A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures - PubMed (original) (raw)
A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures
Simin F Rymo et al. PLoS One. 2011.
Abstract
Background: Myeloid cells have been associated with physiological and pathological angiogenesis, but their exact functions in these processes remain poorly defined. Monocyte-derived tissue macrophages of the CNS, or microglial cells, invade the mammalian retina before it becomes vascularized. Recent studies correlate the presence of microglia in the developing CNS with vascular network formation, but it is not clear whether the effect is directly caused by microglia and their contact with the endothelium.
Methodology/principal findings: We combined in vivo studies of the developing mouse retina with in vitro studies using the aortic ring model to address the role of microglia in developmental angiogenesis. Our in vivo analyses are consistent with previous findings that microglia are present at sites of endothelial tip-cell anastomosis, and genetic ablation of microglia caused a sparser vascular network associated with reduced number of filopodia-bearing sprouts. Addition of microglia in the aortic ring model was sufficient to stimulate vessel sprouting. The effect was independent of physical contact between microglia and endothelial cells, and could be partly mimicked using microglial cell-conditioned medium. Addition of VEGF-A promoted angiogenic sprouts of different morphology in comparison with the microglial cells, and inhibition of VEGF-A did not affect the microglia-induced angiogenic response, arguing that the proangiogenic factor(s) released by microglia is distinct from VEGF-A. Finally, microglia exhibited oriented migration towards the vessels in the aortic ring cultures.
Conclusions/significance: Microglia stimulate vessel sprouting in the aortic ring cultures via a soluble microglial-derived product(s), rather than direct contact with endothelial cells. The observed migration of microglia towards the growing sprouts suggests that their position near endothelial tip-cells could result from attractive cues secreted by the vessels. Our data reveals a two-way communication between microglia and vessels that depends on soluble factors and should extend the understanding of how microglia promote vascular network formation.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
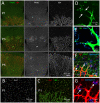
Figure 1. Spatial relationship between microglia and endothelial cells during retinal development in the mouse.
(A) Fluorescence microscopy of IB4 and F4/80 double-stained flat-mounted retinas from newborn mice at postnatal days (P) 1 (P0; day of birth), 3 (P3) and 5 (P5). Note the presence of dispersed IB4 and F4/80 double positive microglial cells ahead of the developing retinal vascular plexus. (B,C) P1 retina double-stained for IB4 and the microglial cell marker Iba1; (B) shows Iba1 staining only, whereas (C) shows double staining. (D–G) Identification of microglia by marker expression and their spatial relationship with tip-cells. Note in (D) how tip-cells (tc) protrude their filopodia towards microglial cells (m). Arrows in (D) point at contacts made between tip-cell filopodia and microglial cytoplasmic protrusions. In (E–G), microglial cells are stained using antibodies against Mac1 and mRNA in situ hybridization for CathepsinS (NBT/BCIP signal is visualized using 633 nm laser reflection). In (F) co-staining of astrocytes for glial fibrillary acidic protein (GFAP) shows the difference in density and distribution between astrocytes and microglia, the latter appearing as solitary cells located within a dense and continuous layer of astrocytes organized in a network structure. In (F,G), which represent the same area at different magnifications and with and without GFAP staining, the left arrow points at a microglial cell situated at a point of contact between two neighboring tip-cells. The right arrow points at a tip-cell extending filopodia towards a microglial cell situated in front of the vascular plexus.
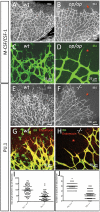
Figure 2. Absence of microglial cells correlates with morphological changes in tip-cells and in the developing retinal vascular plexus.
(A–D) Retinal vascular plexus morphology as depicted by IB4-staining in P6 mice of wildtype (A,C) or csf-1op/op genotype (B,D). Note the complete absence of IB4-stained microglial cells in the retina ahead of the vascular plexus (asterisks), and the difference in vascular density. Note also that the tip-cells and their filopodia in the csf-1op/op retinas (D) are more uniformly radial in their orientation than the corresponding tip-cells of the control (C). (E–H) Retinal vascular plexus morphology as depicted by IB4/Endomucin staining in P6 mice of wildtype (E,G) or Pu.1-/- genotype (F,H). Note the absence of microglial cells (asterisks), the sparser developing vascular plexus and a preferential radial orientation of tip-cell filopodia in the mutants (F,H). (I, J) The maximum angle between the filopodia protruding from a single tip cell is reduced in Pu.1-/- mice (I, n = 76, p<0.0001), and less tip-cells and filopodia were observed in the second row of branches, i.e. at a more central location in the retina of Pu.1-/- mice (J, n = 50, p<0.0001), suggesting that microglia promotes further angiogenic sprouting at this location.
Figure 3. Microglia stimulate angiogenesis.
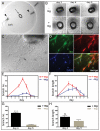
(A) An aortic ring explant (arrow) embedded between two disks of collagen type Ι (col) and surrounded by a cylinder of agarose type VII (aga). Microglia cells in suspension were injected between the discs at two locations (indicated by arrowheads). (B) Phase-contrast images of aortic rings explants from one mouse cultured in the absence (-mg) or in the presence (+mg) of microglia. The images show the same aortic ring explants after three, four and five days in culture. (C) An inverted microscope image showing an aortic ring culture with neovessels at day 4 (arrow points to a vessel). (D) Whole mount immuno-staining of an aortic ring co-cultured with microglia. The aortic ring explant was stained with IB4 (green) for endothelial cells, mouse monoclonal anti-α-smooth muscle actin antibody (red) for pericytes, and DAPI for nucleic acids (blue), and their merged image is shown. (E,F) Numbers and lengths of the vascular branches in 24 aortic ring explants (from four mice) cultured with or without microglia were determined each day for 7 days. The diagrams indicate the mean relative values (the ratio between the particular value and the highest value in the specific series) for number (E) and length (F) of the vascular branches of four independent series of experiments. (G and H) Mean relative values obtained on the day of maximum angiogenic response (day four for cultures in the presence of microglia and at day five for cultures in the absence of microglia) in terms of number (G) and length (H) of the vessels in the four independent experiments above. Bars indicate standard errors of the means. The angiogenic response of aortic rings in terms of branch number was significantly increased in the presence of microglia (***p<0.001), whereas the difference in response in terms of branch length was not statistically significant (ns).
Figure 4. The angiogenic effect of microglial cells is cell type-specific.
(A–C) Mouse aortic ring explants were co-cultured in triplicate with microglial cells (B), mouse embryo fibroblasts (C) or medium only (A). Shown are representative phase contrast microscopy images of the aortic ring cultures at day five. (D) The number of vascular branches generated by the rings was determined and normalized for each series of four independent experiments with four animals. The mean relative number of branches for the 12 rings per condition is presented by the bar graph with standard errors of the means.
Figure 5. The angiogenic stimulatory effect of microglia cells does not depend on direct cell-to-cell contact.
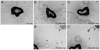
(A–C) Dose-response of microglial cells immobilized in collagen gel in aortic ring cultures. Phase contrast images were obtained at day four for cultures with no added microglia (A), with 25000 microglia cells (B) and with 100000 microglia cells (C). B and C show the central part of the cultures with the aortic ring surrounded by neovessels, while B' and C' also include the embedded microglia cells (arrowheads). Corresponding positions in the respective images are marked with asterisks.
Figure 6. Aortic ring explants induce migration of microglial cells towards the explants.
(A) Aortic ring explants were co-cultured for 12 days with different numbers of microglial cells embedded in collagen and were monitored by phase contrast microscopy. The distance between the aortic ring and the aorta-proximal border of the bulk of microglial cells was measured daily. Images from days 5 and 12 are shown, and stippled white lines indicate the measured decreasing distances. The images in the right panel show the original site of application of the microglial cells, which is marked by a stippled black line. (B,C) Aortic ring explants were co-cultured with mouse embryonic fibroblasts (MEFs) under conditions similar to those in (A). The MEFs exhibited radial growth (arrowhead in B) and, when approaching the vessels, turned away from the aorta ring (arrow in C). (D) Collagen-embedded microglial cells without the presence of an aortic ring spread radially.
Figure 7. The angiogenic response of aortic rings in culture is significantly higher in the presence of activated microglial cells compared with in conditioned medium only.
(A) Phase-contrast images of cultured mouse aortic ring explants with standard culture medium (left), microglia conditioned medium (middle) and with microglia (right). The images show aortic rings at day five of culturing. (B) Diagram showing the mean number of vascular branches in 12 aortic rings for each experimental condition. Conditioned medium was obtained from the same number of microglia as was applied in the co-culture experiment. Bars indicate standard errors of the means. The angiogenic response of aortic rings cultured in the presence of microglia cells was significantly higher than that of aorta rings grown in conditioned medium (p<0.001).
Figure 8. The angiogenic effect of microglia is independent of VEGF-A.
(A–F) Aorta ring explants stimulated with VEGF-A (A and C), VEGF-A plus soluble VEGFR1 (B), microglia (D and F) and microglia plus soluble VEGFR1 (E) are shown by light microscopy. C and F are higher magnification images using differential interference contrast microscopy. Arrows in A and C point to robust microvessels induced by VEGF-A. (G–H) Staining of sprouts with IB4 (green, for endothelial cells) alpha-smooth muscle actin antibodies (ASMA, red, for mural cells) and DAPI (blue, nuclei) alone or in combination. A typical sprout morphology in the presence of VEGF-A is shown in (G) and the corresponding morphology in the presence of microglia is shown in (H).
Similar articles
- VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia.
Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. Gerhardt H, et al. J Cell Biol. 2003 Jun 23;161(6):1163-77. doi: 10.1083/jcb.200302047. Epub 2003 Jun 16. J Cell Biol. 2003. PMID: 12810700 Free PMC article. - SDF-1/CXCR4 contributes to the activation of tip cells and microglia in retinal angiogenesis.
Unoki N, Murakami T, Nishijima K, Ogino K, van Rooijen N, Yoshimura N. Unoki N, et al. Invest Ophthalmol Vis Sci. 2010 Jul;51(7):3362-71. doi: 10.1167/iovs.09-4978. Epub 2010 Feb 24. Invest Ophthalmol Vis Sci. 2010. PMID: 20181837 - Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system.
Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. Gerhardt H, et al. Dev Dyn. 2004 Nov;231(3):503-9. doi: 10.1002/dvdy.20148. Dev Dyn. 2004. PMID: 15376331 - Reciprocal interactions between endothelial cells and macrophages in angiogenic vascular niches.
Baer C, Squadrito ML, Iruela-Arispe ML, De Palma M. Baer C, et al. Exp Cell Res. 2013 Jul 1;319(11):1626-34. doi: 10.1016/j.yexcr.2013.03.026. Epub 2013 Mar 28. Exp Cell Res. 2013. PMID: 23542777 Free PMC article. Review. - How do endothelial cells orientate?
Gerhardt H, Betsholtz C. Gerhardt H, et al. EXS. 2005;(94):3-15. doi: 10.1007/3-7643-7311-3_1. EXS. 2005. PMID: 15617467 Review.
Cited by
- Impact of prenatal immune system disturbances on brain development.
Madhusudan A, Vogel P, Knuesel I. Madhusudan A, et al. J Neuroimmune Pharmacol. 2013 Mar;8(1):79-86. doi: 10.1007/s11481-012-9374-z. Epub 2012 May 13. J Neuroimmune Pharmacol. 2013. PMID: 22580757 Review. - Macrophages and angiogenesis: a role for Wnt signaling.
Newman AC, Hughes CC. Newman AC, et al. Vasc Cell. 2012 Aug 31;4(1):13. doi: 10.1186/2045-824X-4-13. Vasc Cell. 2012. PMID: 22938389 Free PMC article. - Deciphering the roles of macrophages in developmental and inflammation stimulated lymphangiogenesis.
Harvey NL, Gordon EJ. Harvey NL, et al. Vasc Cell. 2012 Sep 3;4(1):15. doi: 10.1186/2045-824X-4-15. Vasc Cell. 2012. PMID: 22943568 Free PMC article. - AS1411 Nucleolin-Specific Binding Aptamers Reduce Pathological Angiogenesis through Inhibition of Nucleolin Phosphorylation.
Iturriaga-Goyon E, Vivanco-Rojas O, Magaña-Guerrero FS, Buentello-Volante B, Castro-Salas I, Aguayo-Flores JE, Gracia-Mora I, Rivera-Huerta M, Sánchez-Bartés F, Garfias Y. Iturriaga-Goyon E, et al. Int J Mol Sci. 2021 Dec 5;22(23):13150. doi: 10.3390/ijms222313150. Int J Mol Sci. 2021. PMID: 34884955 Free PMC article. - LGALS3BP in Microglia Promotes Retinal Angiogenesis Through PI3K/AKT Pathway During Hypoxia.
Zhao C, Liu Y, Meng J, Wang X, Liu X, Li W, Zhou Q, Xiang J, Li N, Hou S. Zhao C, et al. Invest Ophthalmol Vis Sci. 2022 Jul 8;63(8):25. doi: 10.1167/iovs.63.8.25. Invest Ophthalmol Vis Sci. 2022. PMID: 35895036 Free PMC article.
References
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. - PubMed
- Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. - PubMed
- Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. - PubMed
- Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, et al. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous