mTOR couples cellular nutrient sensing to organismal metabolic homeostasis - PubMed (original) (raw)
Review
mTOR couples cellular nutrient sensing to organismal metabolic homeostasis
Jessica J Howell et al. Trends Endocrinol Metab. 2011 Mar.
Abstract
The mammalian target of rapamycin complex 1 (mTORC1) has the ability to sense a variety of essential nutrients and respond by altering cellular metabolic processes. Hence, this protein kinase complex is poised to influence adaptive changes to nutrient fluctuations toward the maintenance of whole-body metabolic homeostasis. Defects in mTORC1 regulation, arising from either physiological or genetic conditions, are believed to contribute to the metabolic dysfunction underlying a variety of human diseases, including type 2 diabetes. We are just now beginning to gain insights into the complex tissue-specific functions of mTORC1. In this review, we detail the current knowledge of the physiological functions of mTORC1 in controlling systemic metabolism, with a focus on advances obtained through genetic mouse models.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
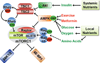
Figure 1. Nutrient sensing and upstream regulation of mTORC1
The presence of extracellular (or systemic) and intracellular nutrients are sensed by mTORC1 and stimulate its activation. In response to insulin, Akt inhibits TSC2, thereby allowing Rheb-GTP to accumulate and activate mTORC1. Nutrients and other factors influencing the energy status of the cell affect intracellular levels of AMP, which activates AMPK. AMPK exerts inhibitory effects on mTORC1 through activation of TSC2 and phosphorylation of Raptor. In addition, the presence of intracellular amino acids is sensed by mTORC1 through an undefined pathway affecting the Rag GTPases. Finally, the two best-characterized direct downstream targets of mTORC1, S6K1/2 and 4E-BP1, are shown.
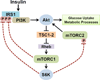
Figure 2. mTORC1 signaling attenuates AKT activation through negative feedback mechanisms
Inhibitory phosphorylation events on IRS1 and the mTORC2 component Rictor by mTORC1 and/or S6K1 suppress the ability of insulin to stimulate Akt, a major metabolic effector of insulin signaling. Through such mechanisms, mTORC1 signaling can decrease insulin sensitivity. Importantly, this feedback effect on AKT signaling has been observed in all metabolic tissues examined (summarized in Table 1).
Similar articles
- Hypothalamic roles of mTOR complex I: integration of nutrient and hormone signals to regulate energy homeostasis.
Hu F, Xu Y, Liu F. Hu F, et al. Am J Physiol Endocrinol Metab. 2016 Jun 1;310(11):E994-E1002. doi: 10.1152/ajpendo.00121.2016. Epub 2016 May 10. Am J Physiol Endocrinol Metab. 2016. PMID: 27166282 Free PMC article. Review. - Influence of mTOR in energy and metabolic homeostasis.
Haissaguerre M, Saucisse N, Cota D. Haissaguerre M, et al. Mol Cell Endocrinol. 2014 Nov;397(1-2):67-77. doi: 10.1016/j.mce.2014.07.015. Epub 2014 Aug 7. Mol Cell Endocrinol. 2014. PMID: 25109278 Review. - Coupling nutrient sensing to metabolic homoeostasis: the role of the mammalian target of rapamycin complex 1 pathway.
André C, Cota D. André C, et al. Proc Nutr Soc. 2012 Nov;71(4):502-10. doi: 10.1017/S0029665112000754. Epub 2012 Aug 9. Proc Nutr Soc. 2012. PMID: 22877732 Review. - [Role of the mTOR pathway in the central regulation of energy balance].
Haissaguerre M, Cota D. Haissaguerre M, et al. Biol Aujourdhui. 2015;209(4):295-307. doi: 10.1051/jbio/2016009. Epub 2016 Mar 28. Biol Aujourdhui. 2015. PMID: 27021048 Review. French. - Mammalian target of rapamycin and tuberous sclerosis complex.
Wataya-Kaneda M. Wataya-Kaneda M. J Dermatol Sci. 2015 Aug;79(2):93-100. doi: 10.1016/j.jdermsci.2015.04.005. Epub 2015 Apr 25. J Dermatol Sci. 2015. PMID: 26051878 Review.
Cited by
- Regulation of blood-testis barrier (BTB) dynamics during spermatogenesis via the "Yin" and "Yang" effects of mammalian target of rapamycin complex 1 (mTORC1) and mTORC2.
Mok KW, Mruk DD, Cheng CY. Mok KW, et al. Int Rev Cell Mol Biol. 2013;301:291-358. doi: 10.1016/B978-0-12-407704-1.00006-3. Int Rev Cell Mol Biol. 2013. PMID: 23317821 Free PMC article. Review. - Transcriptome sequencing and positive selected genes analysis of Bombyx mandarina.
Cheng T, Fu B, Wu Y, Long R, Liu C, Xia Q. Cheng T, et al. PLoS One. 2015 Mar 25;10(3):e0122837. doi: 10.1371/journal.pone.0122837. eCollection 2015. PLoS One. 2015. PMID: 25806526 Free PMC article. - Discovery of small molecule mechanistic target of rapamycin inhibitors as anti-aging and anti-cancer therapeutics.
Chrienova Z, Rysanek D, Oleksak P, Stary D, Bajda M, Reinis M, Mikyskova R, Novotny O, Andrys R, Skarka A, Vasicova P, Novak J, Valis M, Kuca K, Hodny Z, Nepovimova E. Chrienova Z, et al. Front Aging Neurosci. 2022 Dec 6;14:1048260. doi: 10.3389/fnagi.2022.1048260. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36561137 Free PMC article. - Geroconversion: irreversible step to cellular senescence.
Blagosklonny MV. Blagosklonny MV. Cell Cycle. 2014;13(23):3628-35. doi: 10.4161/15384101.2014.985507. Cell Cycle. 2014. PMID: 25483060 Free PMC article. Review. - The Macrophage Switch in Obesity Development.
Castoldi A, Naffah de Souza C, Câmara NO, Moraes-Vieira PM. Castoldi A, et al. Front Immunol. 2016 Jan 5;6:637. doi: 10.3389/fimmu.2015.00637. eCollection 2015. Front Immunol. 2016. PMID: 26779183 Free PMC article. Review.
References
- Wullschleger S, et al. TOR Signaling in Growth and Metabolism. Cell. 2006;124:471–484. - PubMed
- Manning BD, et al. Identification of the Tuberous Sclerosis Complex-2 Tumor Suppressor Gene Product Tuberin as a Target of the Phosphoinositide 3-Kinase/Akt Pathway. Molecular Cell. 2002;10:151–162. - PubMed
- Inoki K, et al. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous