An interferon-induced helicase (IFIH1) gene polymorphism associates with different rates of progression from autoimmunity to type 1 diabetes - PubMed (original) (raw)
An interferon-induced helicase (IFIH1) gene polymorphism associates with different rates of progression from autoimmunity to type 1 diabetes
Christiane Winkler et al. Diabetes. 2011 Feb.
Abstract
Objective: Genome-wide association studies have identified gene regions associated with the development of type 1 diabetes. The aim of this study was to determine whether these associations are with the development of autoimmunity and/or progression to diabetes.
Research design and methods: Children (n = 1,650) of parents with type 1 diabetes were prospectively followed from birth (median follow-up 10.20 years) for the development of islet autoantibodies, thyroid peroxidase antibodies, tissue transglutaminase antibodies, and diabetes. Genotyping for single-nucleotide polymorphisms of the PTPN22, ERBB3, PTPN2, KIAA0350, CD25, and IFIH1 genes was performed using the MassARRAY system with iPLEX chemistry.
Results: Islet autoantibodies developed in 137 children and diabetes developed in 47 children. Type 1 diabetes risk was associated with the IFIH1 rs2111485 single-nucleotide polymorphism (hazard ratio 2.08; 95% CI 1.16-3.74; P = 0.014). None of the other genes were significantly associated with diabetes development in this cohort. IFIH1 genotypes did not associate with the development of islet autoantibodies (P = 0.80) or autoantibodies against thyroid peroxidase (P = 0.55) and tissue transglutaminase (P = 0.66). Islet autoantibody-positive children with the IFIH1 rs2111485 GG genotype had a faster progression to diabetes (31% within 5 years) than children with the type 1 diabetes protective GA or AA genotypes (11% within 5 years; P = 0.006).
Conclusions: The findings indicate that IFIH1 genotypes influence progression from autoimmunity to diabetes development, consistent with the notion that protective genotypes downregulate responses to environmental insults after initiation of autoimmunity.
Figures
FIG. 1.
Cumulative risk for the development of type 1 diabetes by IFIH1 genotypes. A: Children are grouped with respect to IFIH1 SNP rs2111485 genotype into those carrying GG genotype (solid line) and the GA or AA genotype (dashed line). B: Children are grouped by IFIH1 genotypes after stratification of HLA genotypes (solid and dashed lines are children with TEDDY HLA risk genotypes, and the dotted and dot-dashed lines represent children with low-risk HLA genotypes). P values are provided for comparison of IFIH1 GG vs. GA and AA genotypes in the total cohort (A) and for high-risk (P = 0.03) and low-risk (P = 0.06) genotypes. Follow-up (_x_-axis) is from birth. Numbers below the _x_-axis indicate the number of diabetes-free children remaining on follow-up.
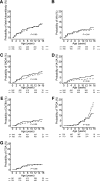
FIG. 2.
Cumulative risk for the development of autoantibodies. Cumulative risk is shown for at least one islet autoantibody (A), IAAs (B), GADAs (C), IA-2As (D), ZnT8As (E), TPOAs (F), and tTGAs (G) by IFIH1 genotypes. Children are grouped with respect to IFIH1 SNP rs2111485 genotype into those carrying the GG genotype (solid line) and the GA or AA genotype (dashed line). Follow-up (_x_-axis) is from birth. Numbers below the _x_-axis indicate the number of autoantibody-negative children remaining on follow-up with respect to age.
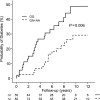
FIG. 3.
Cumulative risk for the progression from islet autoimmunity to type 1 diabetes by IFIH1 genotypes. Islet autoantibody–positive children are grouped with respect to IFIH1 SNP rs2111485 genotype into those carrying the GG genotype (solid line) and the GA or AA genotype (dashed line). Follow-up (_x_-axis) is from the age of the first islet autoantibody–positive sample. Numbers below the _x_-axis indicate the number of diabetes-free children remaining on follow-up.
Similar articles
- A strategy to find gene combinations that identify children who progress rapidly to type 1 diabetes after islet autoantibody seroconversion.
Bonifacio E, Krumsiek J, Winkler C, Theis FJ, Ziegler AG. Bonifacio E, et al. Acta Diabetol. 2014;51(3):403-11. doi: 10.1007/s00592-013-0526-2. Epub 2013 Nov 19. Acta Diabetol. 2014. PMID: 24249616 - Characteristics of rapid vs slow progression to type 1 diabetes in multiple islet autoantibody-positive children.
Achenbach P, Hummel M, Thümer L, Boerschmann H, Höfelmann D, Ziegler AG. Achenbach P, et al. Diabetologia. 2013 Jul;56(7):1615-22. doi: 10.1007/s00125-013-2896-y. Epub 2013 Mar 29. Diabetologia. 2013. PMID: 23539116 - Polymorphisms in the innate immune IFIH1 gene, frequency of enterovirus in monthly fecal samples during infancy, and islet autoimmunity.
Witsø E, Tapia G, Cinek O, Pociot FM, Stene LC, Rønningen KS. Witsø E, et al. PLoS One. 2011;6(11):e27781. doi: 10.1371/journal.pone.0027781. Epub 2011 Nov 14. PLoS One. 2011. PMID: 22110759 Free PMC article. - Effects of type 1 diabetes-associated IFIH1 polymorphisms on MDA5 function and expression.
Looney BM, Xia CQ, Concannon P, Ostrov DA, Clare-Salzler MJ. Looney BM, et al. Curr Diab Rep. 2015 Nov;15(11):96. doi: 10.1007/s11892-015-0656-8. Curr Diab Rep. 2015. PMID: 26385483 Review. - The environment and the origins of islet autoimmunity and Type 1 diabetes.
Eringsmark Regnéll S, Lernmark A. Eringsmark Regnéll S, et al. Diabet Med. 2013 Feb;30(2):155-60. doi: 10.1111/dme.12099. Diabet Med. 2013. PMID: 23252770 Free PMC article. Review.
Cited by
- Enteroviral infections are not associated with type 2 diabetes.
Liu H, Geravandi S, Grasso AM, Sikdar S, Pugliese A, Maedler K. Liu H, et al. Front Endocrinol (Lausanne). 2023 Oct 30;14:1236574. doi: 10.3389/fendo.2023.1236574. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38027145 Free PMC article. - Association of the rs1990760, rs3747517, and rs10930046 polymorphisms in the IFIH1 gene with susceptibility to autoimmune diseases: a meta-analysis.
Xiao Z, Luo S, Zhou Y, Pang H, Yin W, Qin J, Xie Z, Zhou Z. Xiao Z, et al. Front Immunol. 2023 Jun 23;14:1051247. doi: 10.3389/fimmu.2023.1051247. eCollection 2023. Front Immunol. 2023. PMID: 37426657 Free PMC article. - Why does the immune system destroy pancreatic β-cells but not α-cells in type 1 diabetes?
Eizirik DL, Szymczak F, Mallone R. Eizirik DL, et al. Nat Rev Endocrinol. 2023 Jul;19(7):425-434. doi: 10.1038/s41574-023-00826-3. Epub 2023 Apr 18. Nat Rev Endocrinol. 2023. PMID: 37072614 Review. - The longitudinal loss of islet autoantibody responses from diagnosis of type 1 diabetes occurs progressively over follow-up and is determined by low autoantibody titres, early-onset, and genetic variants.
Williams CL, Fareed R, Mortimer GLM, Aitken RJ, Wilson IV, George G, Gillespie KM, Williams AJK; BOX Study Group; Long AE. Williams CL, et al. Clin Exp Immunol. 2022 Dec 15;210(2):151-162. doi: 10.1093/cei/uxac087. Clin Exp Immunol. 2022. PMID: 36181724 Free PMC article. - Non-HLA Gene Polymorphisms in the Pathogenesis of Type 1 Diabetes: Phase and Endotype Specific Effects.
Laine AP, Valta M, Toppari J, Knip M, Veijola R, Ilonen J, Lempainen J. Laine AP, et al. Front Immunol. 2022 Jun 21;13:909020. doi: 10.3389/fimmu.2022.909020. eCollection 2022. Front Immunol. 2022. PMID: 35812428 Free PMC article.
References
- Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 2001;358:221–229 - PubMed
- Schenker M, Hummel M, Ferber K, et al. Early expression and high prevalence of islet autoantibodies for DR3/4 heterozygous and DR4/4 homozygous offspring of parents with type I diabetes: the German BABYDIAB study. Diabetologia 1999;42:671–677 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials