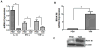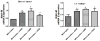TGF-β-induced IRAK-M expression in tumor-associated macrophages regulates lung tumor growth - PubMed (original) (raw)
TGF-β-induced IRAK-M expression in tumor-associated macrophages regulates lung tumor growth
T J Standiford et al. Oncogene. 2011.
Abstract
Tumor-associated macrophages (TAMs) constitute a major component of the immune cell infiltrate observed in the tumor microenvironment (TME). Factors present in the TME, including tumor growth factor-β (TGF-β), allow tumors to circumvent host-mediated immune responses to promote tumor progression. However, the molecular mechanism(s) involved are not clear. Toll-like receptors (TLRs) are important mediators of innate immune responses by immune cells, whose activation triggers the production of molecules required for anti-tumoral responses. Interleukin (IL) receptor-associated kinase (IRAK)-M is an inactive serine/threonine kinase, predominantly expressed in macrophages and is a potent negative regulator of TLR signaling. In this study, we show that TAMs express significantly higher levels of IRAK-M compared with peritoneal macrophages in a syngeneic mouse model of lung cancer. Subcutaneous implantation of Lewis lung carcinoma cells in IRAK-M(-/-) mice resulted in a five-fold reduction in tumor growth as compared with tumors in wild-type (WT) animals. Furthermore, compared with WT TAMs, TAMs isolated from IRAK-M(-/-) mice displayed features of a classically activated (M1) rather than alternatively activated (M2) phenotype, as manifest by greater expression of IL-12, interferon-γ (IFN-γ) and inducible nitric oxide synthase. Human lung cancer cells induced IRAK-M expression in human peripheral blood mononuclear cells (PBMCs) when co-cultured together. Tumor cell-induced expression of IRAK-M was dependent on the activation of TGF-β pathway. Similarly, treatment of human PBMCs or mouse macrophage cell line, RAW 264.4, with TGF-β, induced IRAK-M expression. Interestingly, IRAK-M gene expression in 439 human lung adenocarcinoma tumors correlated with poor survival in patients with lung cancer. Together, our data demonstrates that TGF-β-dependent induction of IRAK-M expression is an important, clinically relevant mechanism by which tumors may circumvent anti-tumor responses of macrophages.
Figures
Figure 1. IL-12, IFN-γ, TNF-α and IRAK-M expression in TAMs and PEMs
TAMs were purified from LLC tumors harvested from CB57/BL6 mice, thioglycolate-elicited PEMs were purified from peritoneal lavage fluid from the same tumor bearing mice. TAMs and PEMs were cultured for 24 h in RPMI 1640 media. Cells were lysed for RNA extraction. IL-12, IFN-γ, TNF-α (A) and IRAK-M (B) expression was assessed by real time PCR. Increase in IRAK-M expression was confirmed at protein level by western immunoblotting (C). Error bars represent SD. statistical significance is assessed by T-test and * denotes p<0.05
Figure 2. Inhibition of syngeneic LLC tumor growth in IRAK-M−/− mice
IRAK-M+/+ (n=6) and IRAK-M−/− mice (n=6) were subcutaneously implanted with 1x 106 LLC cells on either side of the dorsal flank. Tumor growth was monitored by measuring every 5th day for 21 days. (A) Mice were photographed after sacrificing. Tumors harvested from each mouse photographed before further processing. (B) Mean tumor volume for each group (6 mice x 2 tumors each =12) is plotted. Error bars represent SEM. statistical significance is assessed by two sample T-test and * denotes p<0.01
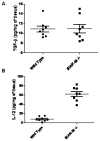
Figure 3. TGF-β and IL-12 protein expression in whole tumor lysates from IRAK-M+/+ and IRAK-M−/− mice
100 mg LLC tumor tissue harvested from IRAK-M+/+ and IRAK-M−/−mice was lysed by sonication in 1 ml of Pierce tissue lysis buffer supplemented with a cocktail of protease inhibitors. Tumor tissue lysates from IRAK-M+/+ (n= 8) and IRAK-M−/− mice (n=8) were assessed for TGF-β (A) and IL-12 (B) proteins by using cytokine specific ELISA. Cytokine levels were expressed as pg/mg of tumor tissue. Error bars represent SEM. Statistical significance is assessed by two sample T-test and * denotes p<0.01
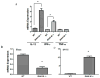
Figure 4. IL-12, IFN-γ TNF-α Fizz1 and iNOS expression in TAMs purified from LLC tumors of IRAK-M+/+ and IRAK-M−/−mice
TAMs were purified from LLC tumors harvested from IRAK-M+/+ and IRAK-M−/−mice and cultured for 24 h in RPMI 1640 media. Cells were lysed for RNA extraction. IL-12, IFN-γ, TNF-α (A), Fizz1(B) and iNOS (C) expression was assessed by real time PCR. Error bars represent SD. Statistical significance is assessed by paired T-test and * denotes p<0.05
Figure 5. TGF-β induces IRAK-M expression in human and murine cells
Human monocytes/macrophages isolated from the blood of a healthy donor (A, B, C) or 24 h serum starved RAW264.7 cells (D) were stimulated with various doses of TGF-β or 10 ng/ml of TGF-β was used to stimulate for different times. Cells were lysed for protein and RNA extraction. IRAK-M mRNA levels were assessed by real time PCR (A and B). Protein expression of IRAK-M was assessed by Western immunoblotting using IRAK-M specific antibody (C and D). Error bars represent SD. Statistical significance is assessed by one-way ANOVA compared to untreated control or 0 h and * denotes p<0.05
Figure 6. Lung cancer cells induce IRAK-M expression in monocytes/macrophages
Different human lung cancer cells were used to stimulate human monocytes/macrophages isolated from the blood of a healthy donor by co-culturing them in either direct contact (A) or in transwell chambers separated by 0.4 micron porous membrane (B). A monocyte to tumor cell ratio of 3:1 was used in all experiments. Cells were lysed for RNA extraction and IRAK-M was assessed by real time PCR. Error bars represent SD. Statistical significance is assessed by one-way ANOVA compared to monocytes alone and * denotes p<0.05
Figure 7. TGF-β augments and TGF-β-neutralizing antibody inhibits tumor cell-induced IRAK-M expression in monocyte/macrophages
In the transwell co-culture system, A549 cells were cultured in the presence or absence of TGF-β (5 ng/ml) in the ratio of 1:3 in the upper chamber and were used to stimulate monocytes/macrophages isolated from the blood of a healthy donor in the lower chamber separated by 0.4 micron porous membrane. After 24 h Monocytes were lysed for RNA extraction and IRAK-M was assessed by real time PCR (A). Error bars represent SD. Statistical significance is assessed by one-way ANOVA compared to monocytes alone and * denotes p<0.05. To assess the effect of TGF-β neutralizing antibody, monocytes/macrophages were cultured in the lower chamber in the presence or absence of TGF-β-neutralizing antibody (50 μg/ml), and stimulated with A549 cells (1:3) in the upper chamber of transwell system. After 24 h Monocytes/macrophages were lysed for protein extraction. IRAK-M protein levels were assessed by Western immunoblotting (B). To demonstrate the efficacy of TGF-β neutralizing antibody, A549 cells were cultured in the absence or presence of indicated concentrations of TGF-β neutralizing antibody for 1 hr. Cells were lysed for protein extraction and TGF-b-induced Smad2 phosphorylation as well as total Smad2 levels were assessed by western immunoblotting (C).
Similar articles
- IRAK-M promotes alternative macrophage activation and fibroproliferation in bleomycin-induced lung injury.
Ballinger MN, Newstead MW, Zeng X, Bhan U, Mo XM, Kunkel SL, Moore BB, Flavell R, Christman JW, Standiford TJ. Ballinger MN, et al. J Immunol. 2015 Feb 15;194(4):1894-904. doi: 10.4049/jimmunol.1402377. Epub 2015 Jan 16. J Immunol. 2015. PMID: 25595781 Free PMC article. - Lack of IL-1 Receptor-Associated Kinase-4 Leads to Defective Th1 Cell Responses and Renders Mice Susceptible to Mycobacterial Infection.
Marinho FV, Fahel JS, Scanga CA, Gomes MT, Guimarães G, Carvalho GR, Morales SV, Báfica A, Oliveira SC. Marinho FV, et al. J Immunol. 2016 Sep 1;197(5):1852-63. doi: 10.4049/jimmunol.1502157. Epub 2016 Jul 20. J Immunol. 2016. PMID: 27439514 - Mycobacterium tuberculosis lipoarabinomannan-mediated IRAK-M induction negatively regulates Toll-like receptor-dependent interleukin-12 p40 production in macrophages.
Pathak SK, Basu S, Bhattacharyya A, Pathak S, Kundu M, Basu J. Pathak SK, et al. J Biol Chem. 2005 Dec 30;280(52):42794-800. doi: 10.1074/jbc.M506471200. Epub 2005 Nov 1. J Biol Chem. 2005. PMID: 16263713 - Tumor-associated macrophages in lung carcinoma: From mechanism to therapy.
Wang X, Wu Y, Gu J, Xu J. Wang X, et al. Pathol Res Pract. 2022 Jan;229:153747. doi: 10.1016/j.prp.2021.153747. Epub 2021 Dec 18. Pathol Res Pract. 2022. PMID: 34952424 Review. - The Role of TGFBR3 in the Development of Lung Cancer.
Deng X, Ma N, He J, Xu F, Zou G. Deng X, et al. Protein Pept Lett. 2024;31(7):491-503. doi: 10.2174/0109298665315841240731060636. Protein Pept Lett. 2024. PMID: 39092729 Review.
Cited by
- A chronic signaling TGFb zebrafish reporter identifies immune response in melanoma.
Noonan HR, Thornock AM, Barbano J, Xifaras ME, Baron CS, Yang S, Koczirka K, McConnell AM, Zon LI. Noonan HR, et al. Elife. 2024 Jun 14;13:e83527. doi: 10.7554/eLife.83527. Elife. 2024. PMID: 38874379 Free PMC article. - The Multifaceted Role of TGF-β in Gastrointestinal Tumors.
Sabbadini F, Bertolini M, De Matteis S, Mangiameli D, Contarelli S, Pietrobono S, Melisi D. Sabbadini F, et al. Cancers (Basel). 2021 Aug 5;13(16):3960. doi: 10.3390/cancers13163960. Cancers (Basel). 2021. PMID: 34439114 Free PMC article. Review. - The Crosstalk Between Tumor-Associated Macrophages (TAMs) and Tumor Cells and the Corresponding Targeted Therapy.
Ge Z, Ding S. Ge Z, et al. Front Oncol. 2020 Nov 3;10:590941. doi: 10.3389/fonc.2020.590941. eCollection 2020. Front Oncol. 2020. PMID: 33224886 Free PMC article. Review. - Anti-tumour strategies aiming to target tumour-associated macrophages.
Tang X, Mo C, Wang Y, Wei D, Xiao H. Tang X, et al. Immunology. 2013 Feb;138(2):93-104. doi: 10.1111/imm.12023. Immunology. 2013. PMID: 23113570 Free PMC article. Review. - TGF-β and the Tissue Microenvironment: Relevance in Fibrosis and Cancer.
Caja L, Dituri F, Mancarella S, Caballero-Diaz D, Moustakas A, Giannelli G, Fabregat I. Caja L, et al. Int J Mol Sci. 2018 Apr 26;19(5):1294. doi: 10.3390/ijms19051294. Int J Mol Sci. 2018. PMID: 29701666 Free PMC article. Review.
References
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. - PubMed
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. - PubMed
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. - PubMed
- Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol. 2006;16:38–52. - PubMed
- Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA132571/CA/NCI NIH HHS/United States
- R01 HL097564/HL/NHLBI NIH HHS/United States
- HL097564/HL/NHLBI NIH HHS/United States
- R01 CA132571-01/CA/NCI NIH HHS/United States
- HL25243/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical