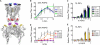Dual-mode phospholipid regulation of human inward rectifying potassium channels - PubMed (original) (raw)
Dual-mode phospholipid regulation of human inward rectifying potassium channels
Wayland W L Cheng et al. Biophys J. 2011.
Abstract
The lipid bilayer is a critical determinant of ion channel activity; however, efforts to define the lipid dependence of channel function have generally been limited to cellular expression systems in which the membrane composition cannot be fully controlled. We reconstituted purified human Kir2.1 and Kir2.2 channels into liposomes of defined composition to study their phospholipid dependence of activity using (86)Rb(+) flux and patch-clamp assays. Our results demonstrate that Kir2.1 and Kir2.2 have two distinct lipid requirements for activity: a specific requirement for phosphatidylinositol 4,5-bisphosphate (PIP(2)) and a nonspecific requirement for anionic phospholipids. Whereas we previously showed that PIP(2) increases the channel open probability, in this work we find that activation by POPG increases both the open probability and unitary conductance. Oleoyl CoA potently inhibits Kir2.1 by antagonizing the specific requirement for PIP(2), and EPC appears to antagonize activation by the nonspecific anionic requirement. Phosphatidylinositol phosphates can act on both lipid requirements, yielding variable and even opposite effects on Kir2.1 activity depending on the lipid background. Mutagenesis experiments point to the role of intracellular residues in activation by both PIP(2) and anionic phospholipids. In conclusion, we utilized purified proteins in defined lipid membranes to quantitatively determine the phospholipid requirements for human Kir channel activity.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
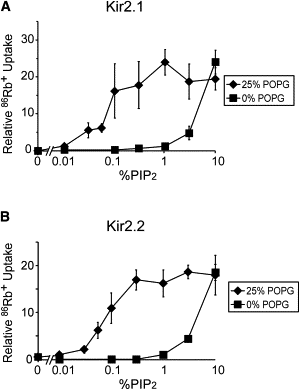
Figure 1
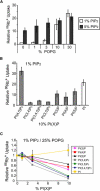
Reconstituted human Kir2.1 and Kir2.2 activity is regulated by POPG. (A) Kir2.1 activity-% PIP2 relationship obtained from 86Rb+ uptake counts at 15 min (all counts are reported from this time point), in 25% POPG or POPE-only liposomes (n = 3, mean ± SE). The valinomycin-normalized counts are plotted relative to counts in 0.01% PIP2 and 25% POPG. (B) Same as in A except for Kir2.2, and 86Rb+ uptake counts taken at 20 min (for POPE-only liposomes, n = 3, mean ± SE).
Figure 2
Kir2.1 has a secondary, nonspecific requirement for anionic phospholipids. (A) 86Rb+ uptake counts of Kir2.1 in liposomes composed of increasing % of the indicated lipid and 1% PIP2 (n = 3, mean ± SE). The valinomycin-normalized counts are plotted relative to counts in 1% PIP2. The asterisks indicate conditions in which liposomes failed to form. 86Rb+ uptake counts for 3%, 10%, and 30% POPG, POPS, POPA, PI, CL, and DGS-NTA are statistically different from control (p < 0.05). (B) 86Rb+ uptake counts of Kir2.1 in liposomes composed of POPE and increasing % of the indicated lipid. The valinomycin-normalized counts are plotted relative to counts in 10% PIP2 (n = 3, mean ± SE).
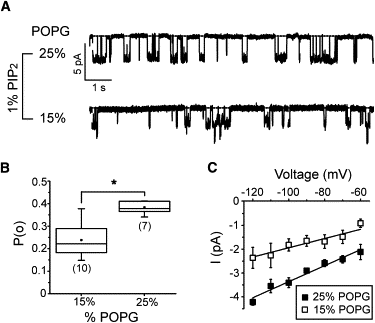
Figure 3
Secondary anionic phospholipid activation increases open probability and unitary conductance. (A) Representative continuous recordings of Kir2.1 currents at −100 mV from giant liposomes composed of the indicated % POPG and 1% PIP2. (B) Box-and-whisker plot of measured Kir2.1 open probabilities from giant liposomes composed of 1% PIP2 and either 15% or 25% POPG. The mean open probabilities, represented by the square box, are 0.24 ± 0.02 for 0.15% POPG and 0.38 ± 0.01 for 25% POPG (mean ± SE). The number of recordings for each condition is indicated in brackets, the whiskers indicate the data range, the box shows the upper and lower quartile values and median, and the asterisk indicates statistical significance (p < 0.05). (C) Current-voltage plot of Kir2.1 analyzed from the same recordings as in B. The derived slope conductances are 19.4 ± 0.6 pS for 15% POPG and 34.5 ± 1.5 pS for 1% PIP2 (mean ± SE).
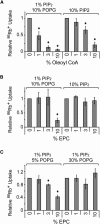
Figure 4
Effects of oleoyl CoA and EPC on Kir2.1 activity. (A) 86Rb+ uptake counts of Kir2.1 in liposomes with increasing % oleoyl CoA and the indicated lipids shown above the graph (n = 3, mean ± SE). Valinomycin-normalized counts are plotted relative to counts in 0% oleoyl CoA. Asterisk indicates a statistically significant difference compared with 0% oleoyl CoA (p < 0.05). (B) Same as in A except with increasing % EPC (n = 3, mean ± SE). Asterisk indicates a statistically significant difference compared with 0% EPC (p < 0.05). (C) Same as B except with a background lipid composition of 1% PIP2 and 5% POPG or 1% PIP2 and 30% POPG (n = 3, mean ± SE).
Figure 5
PIPs act on both modes of lipid regulation. (A) 86Rb+ uptake counts of Kir2.1 in liposomes composed of increasing % POPG and 5% PIP2 (n = 3, mean ± SE). POPG dependence from Fig. 3_A_ is shown in white. Valinomycin-normalized counts are plotted relative to counts in 1% PIP2. (_B)86Rb+ uptake counts of Kir2.1 in liposomes composed of 1% PIP2 and 10% of the indicated lipids (n = 3, mean ± SE). Valinomycin-normalized counts are plotted relative to counts in 1% PIP2 only, and PI data in dashed lines are from Fig. 2_A. 86Rb+ uptake at 10% PI(4,5)P2 is statistically different from all other PI(X)Ps (p < 0.05). (C) 86Rb+ uptake counts of Kir2.1 in liposomes composed of 1% PIP2, 25% POPG, and increasing % of the indicated lipids (n = 3, mean ± SE). Valinomycin-normalized counts are plotted relative to counts in 1% PIP2 and 25% POPG. The difference in 86Rb+ uptake between 10% PI, single-phosphorylated PIPs, and multiphosphorylated PIPs is statistically significant (p < 0.05).
Figure 6
Activation by anionic phospholipids is dependent on intracellular basic residues. (A) Homology model structure of Kir2.1 derived from the Kir2.2 crystal structure (3JYC). Space-filling representations of amino acid side chains are shown for K117 (blue), K120 (black), K182 (red), R189 (pink), and K219 (yellow). (B) 86Rb+ uptake counts of Kir2.1 mutants K117A, K120A, and K117A/K120A in liposomes composed of increasing % PIP2 and 25% POPG (n = 3, mean ± SE). Valinomycin-normalized counts are plotted relative to counts in 0.01% PIP2 and 25% POPG. WT Kir2.1 PIP2 dependence from Fig. 1_A_ is shown in gray with dotted lines (C) Same as in B except in liposomes composed of 1% PIP2 and increasing % POPG. Valinomycin-normalized counts are plotted relative to counts in 1% PIP2 and 0% POPG. WT Kir2.1 POPG dependence from Fig. 2_A_. (D and E) Same as in B and C except for the Kir2.1 mutants K182Q, R189Q, and K219Q (n = 3, mean ± SE).
Similar articles
- Direct and specific activation of human inward rectifier K+ channels by membrane phosphatidylinositol 4,5-bisphosphate.
D'Avanzo N, Cheng WW, Doyle DA, Nichols CG. D'Avanzo N, et al. J Biol Chem. 2010 Nov 26;285(48):37129-32. doi: 10.1074/jbc.C110.186692. Epub 2010 Oct 4. J Biol Chem. 2010. PMID: 20921230 Free PMC article. - Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2.
Hansen SB, Tao X, MacKinnon R. Hansen SB, et al. Nature. 2011 Aug 28;477(7365):495-8. doi: 10.1038/nature10370. Nature. 2011. PMID: 21874019 Free PMC article. - Structural basis of control of inward rectifier Kir2 channel gating by bulk anionic phospholipids.
Lee SJ, Ren F, Zangerl-Plessl EM, Heyman S, Stary-Weinzinger A, Yuan P, Nichols CG. Lee SJ, et al. J Gen Physiol. 2016 Sep;148(3):227-37. doi: 10.1085/jgp.201611616. Epub 2016 Aug 15. J Gen Physiol. 2016. PMID: 27527100 Free PMC article. - How highly charged anionic lipids bind and regulate ion channels.
Tucker SJ, Baukrowitz T. Tucker SJ, et al. J Gen Physiol. 2008 May;131(5):431-8. doi: 10.1085/jgp.200709936. Epub 2008 Apr 14. J Gen Physiol. 2008. PMID: 18411329 Free PMC article. Review. No abstract available. - Regulation of cardiac inwardly rectifying potassium channels by membrane lipid metabolism.
Takano M, Kuratomi S. Takano M, et al. Prog Biophys Mol Biol. 2003 Jan;81(1):67-79. doi: 10.1016/s0079-6107(02)00048-2. Prog Biophys Mol Biol. 2003. PMID: 12475570 Review.
Cited by
- The versatile regulation of K2P channels by polyanionic lipids of the phosphoinositide and fatty acid metabolism.
Riel EB, Jürs BC, Cordeiro S, Musinszki M, Schewe M, Baukrowitz T. Riel EB, et al. J Gen Physiol. 2022 Feb 7;154(2):e202112989. doi: 10.1085/jgp.202112989. Epub 2021 Dec 20. J Gen Physiol. 2022. PMID: 34928298 Free PMC article. - Cooperative Gating of a K+ Channel by Unmodified Biological Anionic Lipids Viewed by Solid-State NMR Spectroscopy.
Yekefallah M, van Aalst EJ, van Beekveld RAM, Eason IR, Breukink E, Weingarth M, Wylie BJ. Yekefallah M, et al. J Am Chem Soc. 2024 Feb 21;146(7):4421-4432. doi: 10.1021/jacs.3c09266. Epub 2024 Feb 9. J Am Chem Soc. 2024. PMID: 38334076 Free PMC article. - Differential lipid dependence of the function of bacterial sodium channels.
D'Avanzo N, McCusker EC, Powl AM, Miles AJ, Nichols CG, Wallace BA. D'Avanzo N, et al. PLoS One. 2013 Apr 8;8(4):e61216. doi: 10.1371/journal.pone.0061216. Print 2013. PLoS One. 2013. PMID: 23579615 Free PMC article. - Selective binding of a toxin and phosphatidylinositides to a mammalian potassium channel.
Liu Y, LoCaste CE, Liu W, Poltash ML, Russell DH, Laganowsky A. Liu Y, et al. Nat Commun. 2019 Mar 22;10(1):1352. doi: 10.1038/s41467-019-09333-4. Nat Commun. 2019. PMID: 30902995 Free PMC article. - Enantioselective protein-sterol interactions mediate regulation of both prokaryotic and eukaryotic inward rectifier K+ channels by cholesterol.
D'Avanzo N, Hyrc K, Enkvetchakul D, Covey DF, Nichols CG. D'Avanzo N, et al. PLoS One. 2011 Apr 29;6(4):e19393. doi: 10.1371/journal.pone.0019393. PLoS One. 2011. PMID: 21559361 Free PMC article.
References
- Hilgemann D.W., Ball R. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science. 1996;273:956–959. - PubMed
- Huang C.L., Feng S.Y., Hilgemann D.W. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. - PubMed
- Ma H.P., Saxena S., Warnock D.G. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC) J. Biol. Chem. 2002;277:7641–7644. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources