Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor - PubMed (original) (raw)
. 2011 Feb 15;108(7):2807-12.
doi: 10.1073/pnas.1019761108. Epub 2011 Jan 31.
Li Pan, Xiao-man Yang, Virginia C Hughes, Johnathon R Walls, Melissa G Dominguez, Mary V Simmons, Patricia Burfeind, Yingzi Xue, Yi Wei, Lynn E Macdonald, Gavin Thurston, Christopher Daly, Hsin Chieh Lin, Aris N Economides, David M Valenzuela, Andrew J Murphy, George D Yancopoulos, Nicholas W Gale
Affiliations
- PMID: 21282641
- PMCID: PMC3041062
- DOI: 10.1073/pnas.1019761108
Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor
Keith D Anderson et al. Proc Natl Acad Sci U S A. 2011.
Abstract
The vasculature of the CNS is structurally and functionally distinct from that of other organ systems and is particularly prone to developmental abnormalities and hemorrhage. Although other embryonic tissues undergo primary vascularization, the developing nervous system is unique in that it is secondarily vascularized by sprouting angiogenesis from a surrounding perineural plexus. This sprouting angiogenesis requires the TGF-β and Wnt pathways because ablation of these pathways results in aberrant sprouting and hemorrhage. We have genetically deleted Gpr124, a member of the large family of long N-terminal group B G protein-coupled receptors, few members of which have identified ligands or well-defined biologic functions in mammals. We show that, in the developing CNS, Gpr124 is specifically expressed in the vasculature and is absolutely required for proper angiogenic sprouting into the developing neural tube. Embryos lacking Gpr124 exhibit vascular defects characterized by delayed vascular penetration, formation of pathological glomeruloid tufts within the CNS, and hemorrhage. In addition, they display defects in palate and lung development, two processes in which TGF-β and/or Wnt pathways also play important roles. We also show that TGF-β stimulates Gpr124 expression, and ablation of Gpr124 results in perturbed TGF-β pathway activation, suggesting roles for Gpr124 in modulating TGF-β signaling. These results represent a unique function attributed to a long N-terminal group B-type G protein-coupled receptor in a mammalian system.
Conflict of interest statement
Conflict of interest statement: The authors are employees of Regeneron Pharmaceuticals, Inc.
Figures
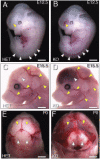
Fig. 1.
Targeted deletion of Gpr124 results in profound CNS-specific vascular hemorrhage. Gpr124Lz/Lz (KO) mutants displayed prominent hemorrhage in the ventral forebrain and along the spinal cord at E12.5 [arrowheads indicate hemorrhage in Gpr124Lz/Lz mutants (B) and corresponding normal regions in heterozygous (Het) Gpr124Lz/WT embryos (A)]. The hemorrhage extended throughout the forebrain of KO mutants at E15 (white arrowhead in D) and through to birth (P0) (yellow arrowheads in F). (Compare with corresponding normal regions in Het embryos in C and E). Stereotypic, large superficial vessels of the head and forelimb had the same normal appearance in KO mutants as in Het embryos (yellow arrowheads in C and D).
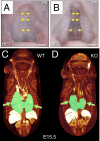
Fig. 2.
Failure in secondary palate formation and hypoplasia of lungs in Gpr124-deficient embryos. All Gpr124-deficient embryos had a large cleft of the secondary hard palate (B), which is normally fused (A), at P0. Yellow arrowheads point to the fused midline in A and to the unfused medial edges of the palatal shelves in B. Visualization of embryonic morphology by soft tissue–enhanced microcomputed X-ray tomography (μCT) at E15.5 revealed that, although other internal organs appeared normal, lungs (shaded green, arrows) were hypoplastic in Gpr124-deficient embryos (D) compared with control littermates (C).
Fig. 3.
Gpr124 is required for normal angiogenic sprouting and development of vasculature within the forebrain and spinal cord. Endothelial immunostaining for platelet endothelial cell adhesion molecule 1 (PECAM-1) (A_–_D and F_–_I) showed that, although a PNVP formed in the forebrain of both control Gpr124Lz/WT (Het) embryos and Gpr124Lz/Lz (KO) embryos (blue arrowheads in A and B), angiogenic sprouting into the ventral forebrain was evident only in Het embryos at E10.5 (yellow arrowheads in A and B). Similarly, vessel sprouting into the spinal cord (yellow arrowheads in F and G) from the PNVP (blue arrowheads in F and G) was reduced in the dorsal spinal cord and absent in the ventral spinal cord of KO embryos at E10.5. Vessels were grossly aberrant in the ventral forebrain in KO embryos at E12.5, featuring glomeruloid tuft–like endings (yellow arrowheads in D); large regions in the periventricular portion of the ganglionic eminences, and in the lateral pallium, remained avascular. A thickening of the PNVP often occurred along the lateral pallium (blue arrowheads in C and D). Abnormal vessel formations also were present in the ventral spinal cord of KO embryos at E12.5 (yellow arrowheads in I). Histochemical staining for endogenous peroxidase contained in red blood cells (E and J) clearly demonstrated accumulation of red blood cells in neural tissue surrounding abnormal vessels in both brain and ventral spinal cord as well as in the central canal (arrowheads in E and J). Sections in E and J were counterstained with eosin. VFB, ventral forebrain; MGE, medial ganglionic eminence; LGE, lateral ganglionic eminence.
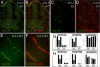
Fig. 4.
Gpr124 is expressed in normal developing CNS vessels of E10.5 and E12.5 embryos. Fluorescent RNA in situ hybridization with a probe for Gpr124 (green) coupled with fluorescent histochemical staining of endothelial cells by GS lectin I (red) in cross-sections through the neural tube of E10.5 WT embryos (A and B) showed Gpr124 expression within the developing CNS vasculature. Gpr124 expression was not detected in Gp124 KO embryos (C and D). Gpr124 (green) continued to be expressed within the vasculature of the CNS in WT E12.5 embryos (E and F). RT-PCR measurements on platelet endothelial cell adhesion molecule–positive endothelial cells and PDGF receptor β–positive pericytes isolated by FACS from the brains of WT embryos at E15.5 demonstrated that both endothelial cells (EC) and pericytes (PC) have enriched expression of Gpr124 relative to nonendothelial cells [“flow through” (FT)] and nonpericyte cells (FT), respectively (G and H). Control probes for VEGF receptor 2, PDGF receptor β, and cyclophilin confirmed the endothelial identity of isolated cells, the pericyte identity of isolated cells, and the integrity of mRNA samples, respectively. The y axis scale is proportional to RNA copy number.
Fig. 5.
Microarray analysis of alterations in embryonic brain and vascular gene expression resulting from global deletion of Gpr124. The effect of deleting Gpr124 on gene expression is shown for sets of genes representing TGF-β target genes (A), β-catenin target genes (B), and Notch target genes (C). The analysis was performed on mRNA extracted from isolated samples of vessels from the ventral forebrain and samples of ventral forebrain neuroepithelium (excluding vessels) at E12.5 collected by laser microdissection. Values are mean fold change in Gpr124Lz/Lz (KO) samples versus Gpr124WT/WT (WT) samples (n = 3–4 KO embryos; n = 3 WT embryos). Only values with a mean fold change >1.5, or <0.66, and a KO vs. WT t test P value ≤ 0.05 are shown. Values not meeting both of these criteria were considered to be unchanged and are represented by dots. Genes represented more than once showed significant changes in expression by multiple probes.
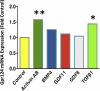
Fig. 6.
Gpr124 mRNA expression is induced by TGF-β1 in HUVECs. Treatment of HUVECs for 6 h with 50 pM TGF-β1 induced a 1.44-fold increase in expression of Gpr124 mRNA, as measured by microarray. Treatment with 600 pM activin AB induced a 1.58-fold increase. Values are the mean of three replicates. *P < 0.05; **P < 0.01.
Similar articles
- Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling.
Zhou Y, Nathans J. Zhou Y, et al. Dev Cell. 2014 Oct 27;31(2):248-56. doi: 10.1016/j.devcel.2014.08.018. Epub 2014 Oct 16. Dev Cell. 2014. PMID: 25373781 Free PMC article. - GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier.
Cullen M, Elzarrad MK, Seaman S, Zudaire E, Stevens J, Yang MY, Li X, Chaudhary A, Xu L, Hilton MB, Logsdon D, Hsiao E, Stein EV, Cuttitta F, Haines DC, Nagashima K, Tessarollo L, St Croix B. Cullen M, et al. Proc Natl Acad Sci U S A. 2011 Apr 5;108(14):5759-64. doi: 10.1073/pnas.1017192108. Epub 2011 Mar 18. Proc Natl Acad Sci U S A. 2011. PMID: 21421844 Free PMC article. - Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/β-catenin pathway during brain angiogenesis.
Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, Maquet E, Gauquier A, Cabochette P, Fukuhara S, Mochizuki N, Nathans J, Stainier DY. Vanhollebeke B, et al. Elife. 2015 Jun 8;4:e06489. doi: 10.7554/eLife.06489. Elife. 2015. PMID: 26051822 Free PMC article. - The Wnt7's Tale: A story of an orphan who finds her tie to a famous family.
Noda M, Vallon M, Kuo CJ. Noda M, et al. Cancer Sci. 2016 May;107(5):576-82. doi: 10.1111/cas.12924. Epub 2016 Apr 7. Cancer Sci. 2016. PMID: 26934061 Free PMC article. Review. - Vascularisation of the central nervous system.
Tata M, Ruhrberg C, Fantin A. Tata M, et al. Mech Dev. 2015 Nov;138 Pt 1:26-36. doi: 10.1016/j.mod.2015.07.001. Epub 2015 Jul 26. Mech Dev. 2015. PMID: 26222953 Free PMC article. Review.
Cited by
- Transgenic animal models to explore and modulate the blood brain and blood retinal barriers of the CNS.
Goncalves A, Antonetti DA. Goncalves A, et al. Fluids Barriers CNS. 2022 Nov 1;19(1):86. doi: 10.1186/s12987-022-00386-0. Fluids Barriers CNS. 2022. PMID: 36320068 Free PMC article. Review. - Single-cell atlas of the human brain vasculature across development, adulthood and disease.
Wälchli T, Ghobrial M, Schwab M, Takada S, Zhong H, Suntharalingham S, Vetiska S, Gonzalez DR, Wu R, Rehrauer H, Dinesh A, Yu K, Chen ELY, Bisschop J, Farnhammer F, Mansur A, Kalucka J, Tirosh I, Regli L, Schaller K, Frei K, Ketela T, Bernstein M, Kongkham P, Carmeliet P, Valiante T, Dirks PB, Suva ML, Zadeh G, Tabar V, Schlapbach R, Jackson HW, De Bock K, Fish JE, Monnier PP, Bader GD, Radovanovic I. Wälchli T, et al. Nature. 2024 Aug;632(8025):603-613. doi: 10.1038/s41586-024-07493-y. Epub 2024 Jul 10. Nature. 2024. PMID: 38987604 Free PMC article. - Brain endothelial cells acquire blood-brain barrier properties in the absence of Vegf-dependent CNS angiogenesis.
Fetsko AR, Sebo DJ, Taylor MR. Fetsko AR, et al. Dev Biol. 2023 Feb;494:46-59. doi: 10.1016/j.ydbio.2022.11.007. Epub 2022 Dec 9. Dev Biol. 2023. PMID: 36502932 Free PMC article. - Developmental and pathological angiogenesis in the central nervous system.
Vallon M, Chang J, Zhang H, Kuo CJ. Vallon M, et al. Cell Mol Life Sci. 2014 Sep;71(18):3489-506. doi: 10.1007/s00018-014-1625-0. Epub 2014 Apr 24. Cell Mol Life Sci. 2014. PMID: 24760128 Free PMC article. Review. - The inner blood-retinal barrier: Cellular basis and development.
Díaz-Coránguez M, Ramos C, Antonetti DA. Díaz-Coránguez M, et al. Vision Res. 2017 Oct;139:123-137. doi: 10.1016/j.visres.2017.05.009. Epub 2017 Jun 27. Vision Res. 2017. PMID: 28619516 Free PMC article. Review.
References
- Hogan KA, Ambler CA, Chapman DL, Bautch VL. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development. 2004;131:1503–1513. - PubMed
- Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res. 2003;314:119–129. - PubMed
- Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases