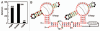Bacterial aptamers that selectively bind glutamine - PubMed (original) (raw)
Bacterial aptamers that selectively bind glutamine
Tyler D Ames et al. RNA Biol. 2011 Jan-Feb.
Abstract
The continued expansion of microbial sequence data has allowed for the detection of an increasing number of conserved RNA motifs by using comparative sequence analysis. Recently, we reported the discovery of two structured non-coding RNA motifs, called glnA and Downstream-peptide, that have similarity in sequence and secondary structure. In this report, we describe data demonstrating that representatives of both RNA motifs selectively bind the amino acid L-glutamine. These glutamine aptamers are found exclusively in cyanobacteria and marine metagenomic sequences, wherein several glnA RNA representatives reside upstream of genes involved in nitrogen metabolism. These motifs have genomic distributions that are consistent with a gene regulation function, suggesting they are components of glutamine-responsive riboswitches. Thus, our findings implicate glutamine as a regulator of cyanobacterial nitrogen metabolism pathways. Furthermore, our findings expand the collection of natural aptamer classes that bind amino acids to include glycine, lysine and glutamine.
Figures
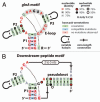
Figure 1
Consensus sequence and secondary structure models for two candidate riboswitch aptamer families. (A) The glnA motif is a 3-stem junction (stems are named P1, P2 and P3) that carries an E-loop and a possible single long-distance base pair (dashed line). (B) The Downstream-peptide motif is formed by three extended base-paired substructures wherein P1 and P2 are nearly identical to those of the glnA motif. The motif lacks P3 and E-loop features, but nucleotides in this region form a pseudoknot. Like glnA RNAs, the Downstream-peptide motif can potentially form a single long-range base pair. The two motifs also carry identical nucleotides at the base of P1 and in the junction. These models are derived using methods and data reported previously in reference .
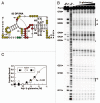
Figure 2
The 67 glnA RNA binds
l
-glutamine. (A) Sequence and secondary structural model for the 67 glnA RNA from S. elongatus. Circled positions indicate areas of the RNA where internucleotide linkages undergo reduced (red) or constant (yellow) scission as ligand concentrations are increased when subjected to in-line probing (data from B). Nucleotides depicted in lowercase identify guanosine residues added to the construct to facilitate efficient in vitro transcription. Asterisks indicate the boundaries of the annotations for in-line probing results that could be clearly resolved by PAGE. (B) In-line probing analysis of 5′ 32P-labeled 67 glnA RNA. Precursor RNAs (Pre) were loaded onto gel lanes after treatment as follows: NR, no reaction; T1, partial digest with RNase T1 (cleaves after G residues); −OH, partial alkaline-mediated degradation. Additional lanes were loaded with precursor RNAs subjected to in-line probing conditions without ligand (-), or were subjected to inline probing conditions in the presence of various concentrations of
l
-glutamine ranging from 1 µM to 10 mM. Vertical lines designate areas where band intensities decrease as the RNA is exposed to higher concentrations of ligand. Band intensities of numbered regions were quantified and used to assess the extent of ligand binding. (C) Plot of the normalized fraction of band modulation (interpreted as fraction of RNAs bound to ligand) versus the logarithm of the concentration of ligand. Regions are as depicted in (B). The line represents the curve expected for a 1-to-1 RNA-ligand interaction with a _K_D of 575 µM.
Figure 3
The 67 glnA RNA binds L-glutamine but rejects a variety of other compounds. (A) In-line probing analysis of the 67 glnA RNA with several glutamine analogs. Precursor RNAs were subjected to in-line probing conditions in the presence of 10 mM L-glutamine (L-gln) or 10 mM of the various compounds as follows: Ala-Gln (1), L-glutamine t-butyl ester (2), L-theanine (3), O-acetyl-L-serine (4), L-homoglutamine (5), L-β-homoglutamine (6), (S)-2-amino-5-oxo-hexonic acid (7), 5-amino-oxopentanoic acid (8) and asparagine (9). Other annotations are as described for Figure 2B. (B) Chemical structures of compounds tested with both the 67 glnA and 83 DP RNAs.
Figure 4
Tandem glutamine aptamers. (A) Distribution of glutamine aptamers among single, double and triple arrangements. Aptamers were grouped together if the amount of intervening sequence was less than 100 nucleotides. (B) Consensus sequence and structure of the most common tandem arrangement of glutamine aptamers. Annotations are as described in Figure 1A.
Figure 5
The 83 DP RNA is an aptamer for glutamine. (A) Sequence and predicted secondary structure of the 83 DP RNA. Nucleotides circled in green indicate internucleotide linkages that undergo greater scission when the RNA is exposed to ligand. Other annotations are as described for Figure 2A. (B) In-line probing analysis of 5′ 32P-labeled 83 DP RNA with various concentrations of L-glutamine ranging from 10 µM to 10 mM. Annotations are as described for Figure 2B, with the exception that arrows indicate specific bands which were used to make the _K_D plot in (C). (C) Plot representing ligand binding as described for Figure 2C. The line represents the curve expected for a 1-to-1 interaction using a _K_D value 5 mM.
Similar articles
- Guanine riboswitch variants from Mesoplasma florum selectively recognize 2'-deoxyguanosine.
Kim JN, Roth A, Breaker RR. Kim JN, et al. Proc Natl Acad Sci U S A. 2007 Oct 9;104(41):16092-7. doi: 10.1073/pnas.0705884104. Epub 2007 Oct 2. Proc Natl Acad Sci U S A. 2007. PMID: 17911257 Free PMC article. - Role of GlnR in Controlling Expression of Nitrogen Metabolism Genes in Listeria monocytogenes.
Biswas R, Sonenshein AL, Belitsky BR. Biswas R, et al. J Bacteriol. 2020 Sep 8;202(19):e00209-20. doi: 10.1128/JB.00209-20. Print 2020 Sep 8. J Bacteriol. 2020. PMID: 32690554 Free PMC article. - A eubacterial riboswitch class that senses the coenzyme tetrahydrofolate.
Ames TD, Rodionov DA, Weinberg Z, Breaker RR. Ames TD, et al. Chem Biol. 2010 Jul 30;17(7):681-5. doi: 10.1016/j.chembiol.2010.05.020. Chem Biol. 2010. PMID: 20659680 Free PMC article. - Biophysical Approaches to Bacterial Gene Regulation by Riboswitches.
Perez-Gonzalez C, Grondin JP, Lafontaine DA, Carlos Penedo J. Perez-Gonzalez C, et al. Adv Exp Med Biol. 2016;915:157-91. doi: 10.1007/978-3-319-32189-9_11. Adv Exp Med Biol. 2016. PMID: 27193543 Review. - Metabolite and light regulation of metabolism in plants: lessons from the study of a single biochemical pathway.
Oliveira IC, Brenner E, Chiu J, Hsieh MH, Kouranov A, Lam HM, Shin MJ, Coruzzi G. Oliveira IC, et al. Braz J Med Biol Res. 2001 May;34(5):567-75. doi: 10.1590/s0100-879x2001000500003. Braz J Med Biol Res. 2001. PMID: 11323742 Review.
Cited by
- Structure and ligand binding of the glutamine-II riboswitch.
Huang L, Wang J, Watkins AM, Das R, Lilley DMJ. Huang L, et al. Nucleic Acids Res. 2019 Aug 22;47(14):7666-7675. doi: 10.1093/nar/gkz539. Nucleic Acids Res. 2019. PMID: 31216023 Free PMC article. - Prospects for riboswitch discovery and analysis.
Breaker RR. Breaker RR. Mol Cell. 2011 Sep 16;43(6):867-79. doi: 10.1016/j.molcel.2011.08.024. Mol Cell. 2011. PMID: 21925376 Free PMC article. Review. - The structure of a tetrahydrofolate-sensing riboswitch reveals two ligand binding sites in a single aptamer.
Trausch JJ, Ceres P, Reyes FE, Batey RT. Trausch JJ, et al. Structure. 2011 Oct 12;19(10):1413-23. doi: 10.1016/j.str.2011.06.019. Epub 2011 Sep 8. Structure. 2011. PMID: 21906956 Free PMC article. - Modulation of quaternary structure and enhancement of ligand binding by the K-turn of tandem glycine riboswitches.
Baird NJ, Ferré-D'Amaré AR. Baird NJ, et al. RNA. 2013 Feb;19(2):167-76. doi: 10.1261/rna.036269.112. Epub 2012 Dec 17. RNA. 2013. PMID: 23249744 Free PMC article. - Atomic resolution mechanistic studies of ribocil: A highly selective unnatural ligand mimic of the E. coli FMN riboswitch.
Howe JA, Xiao L, Fischmann TO, Wang H, Tang H, Villafania A, Zhang R, Barbieri CM, Roemer T. Howe JA, et al. RNA Biol. 2016 Oct 2;13(10):946-954. doi: 10.1080/15476286.2016.1216304. Epub 2016 Aug 2. RNA Biol. 2016. PMID: 27485612 Free PMC article.
References
- Loh E, Dussurget O, Gripenland J, Vaitkevicius K, Tiensuu T, Mandin P, et al. A trans-acting riboswitch controls expression of the virulence regulator PrfA in listeria monocytogenes. Cell. 2009;139:770–779. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases