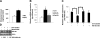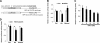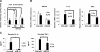Cholesterol regulation of receptor-interacting protein 140 via microRNA-33 in inflammatory cytokine production - PubMed (original) (raw)
Cholesterol regulation of receptor-interacting protein 140 via microRNA-33 in inflammatory cytokine production
Ping-Chih Ho et al. FASEB J. 2011 May.
Abstract
Receptor interacting protein 140 (RIP140) is a nuclear receptor coregulator that affects a wide spectrum of biological processes. It is unclear whether and how the expression level of RIP140 can be modulated and whether RIP140 is involved in inflammatory diseases. Here, we examine how intracellular cholesterol regulates RIP140 expression, and we evaluate the effect of RIP140 expression on macrophage proinflammatory potential. Macrophages treated with modified low-density lipoprotein express higher RIP140 mRNA and protein levels. Consistently, simvastatin reduces RIP140 levels, which can be reversed by mevalonate. Moreover, a high-fat diet elevates RIP140 but lowers miR-33 levels in peritoneal macrophages, and increases the production of IL-1β and TNF-α in macrophages. Mechanistically, miR-33 targets RIP140 mRNA by recognizing its target located in a highly conserved sequence of the 3'-untranslated region (3'-UTR) of RIP140 mRNA. Consequentially, miR-33 reduces RIP140 coactivator activity for NF-κB, which is supported by the reduction in NF-κB reporter activity and the inflammatory potential in macrophages. This study uncovers a cholesterol-miR-33-RIP140 regulatory pathway that modulates the proinflammatory potential in macrophages in response to an alteration in the intracellular cholesterol status, and identifies RIP140 as a direct target of miR-33 that mediates simvastatin-triggered anti-inflammation.
Figures
Figure 1.
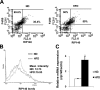
Effect of high-fat diet on RIP140 expression in peritoneal macrophages. A) Primary peritoneal macrophages were obtained from C56BL/6J mice fed either a normal diet (ND) or a high-fat diet (HFD) for 2 wk. RIP140 and F4/80 expression profiles were determined by flow cytometry. The y axis shows the staining intensity of macrophage marker F4/80. The x axis shows the staining intensity of RIP140. B) Histogram showing RIP140 expression from F4/80-positive peritoneal macrophages. Geological means for ND and HFD were indicated. C) Real-time analysis of RIP140 mRNA levels in peritoneal macrophages from animals fed the indicated diets. All values represent means ±
sd
; n = 3. *P < 0.05 vs. normal diet.
Figure 2.
Effect of intracellular cholesterol level on RIP140 expression. A) Intracellular cholesterol contents of primary peritoneal macrophages from ND or HFD. *P < 0.05 vs. normal diet. B) Raw264.7 murine macrophages were treated with vehicle, AcLDL, or OxLDL in the medium for 8 h. mRNA levels of RIP140 were determined by semiquantitative real-time PCR. *P < 0.05 vs. normal diet. C, D) mRNA (C) and protein (D) levels of RIP140 from Raw264.7 murine macrophages treated as indicated for 24 h. Quantified results are shown at bottom of image. mRNA levels were determined by semiquantitative real-time PCR. *P < 0.05. Values represent means ±
sd
; n = 3.
Figure 3.
Simvastatin reduces RIP140 expression, mediated by miR-33. A) Predicted target site of miR-33 located in a conserved 3′-UTR of RIP140 mRNA, conducted by TargetScan. B) Left panel: RIP140 mRNA level. Right panel: mature miR-33 level. Samples were from Raw264.7 macrophages under control or simvastatin treatment for 24 h. C) Real-time analysis of mature miR-33 levels in peritoneal macrophages from mice fed either ND or HFD for 2 wk. D) RIP140 mRNA levels from Raw264.7 macrophages treated with control vehicle or simvastatin in the presence of miR-33 or miR-33 inhibitor. Anti-miR-33:miR-33 inhibitor. E) RIP140 protein levels from Raw264.7 macrophages treated with control vehicle or simvastatin in the presence of miR-33 or miR-33 inhibitor (anti-miR-33). Quantified data are shown at bottom of image. All values show means ±
sd
; n = 3. *P < 0.05.
Figure 4.
miR-33 targets a conserved 3′-UTR of RIP140 mRNA to repress RIP140. A) Schematic diagram of reporter constructs containing the wild-type or mutated 3′-UTR of RIP140 mRNA. B) miR-33 represses the luciferase activity of the wild-type reporter but not the mutant reporter in 293T cells. C) Dose-dependent repressive effects of miR-33 mimic on the wild-type reporter in 293T cells. *P < 0.05 vs. 0 nM. D) Simvastatin suppresses luciferase activity of the wild-type reporter but not the mutant reporter in BV2 cells. Ctrl, control vector; Wt, wild-type 3′-UTR reporter; mutant, 3′-UTR reporter with mutations in the miR-33 target site. *P < 0.05 vs. control treatment. All values show means ±
sd
; n = 3.
Figure 5.
miR-33 modulates inflammatory cytokine production by controlling RIP140 expression. A) Simvastatin suppresses the effect of RIP140 on promoting NF-κB-driven reporter activity in 293T cells. After transfection with plasmids for 12 h, 293T cells were treated with vehicle (Ctrl) or simvastatin for another 24 h, followed by assays of luciferase activity. B) Real-time analysis of mRNA levels of IL-1β and TNF-α expression in Raw264.7 murine macrophages transfected with the control miR or miR-33 in the absence or presence of LPS for 4 h. C) miR-33 reduces IL-1β and TNF-α contents in the culture supernatant of Raw264.7 macrophages in the absence or presence of LPS. Cytokine production was determined by ELISA. All values show means ±
sd
; n = 3. *P < 0.05.
Figure 6.
Expression of RIP140 in macrophages modulates inflammatory cytokines production and acute septic shock. A) Overexpression of RIP140 in Raw264.7 cells up-regulated basal and LPS-induced levels of IL-1β and TNF-α. B) Silencing RIP140 in Raw264.7 macrophages reduced the levels of IL-1β and TNF-α. mRNA, determined by real-time qPCR; n = 3. *P < 0.05 vs. control vector LPS or control siRNA LPS group; **P < 0.05 vs. control vector or control siRNA group. C) HFD reduced the animal survival rate in the acute septic shock model. Difference was significant between ND and HFD in Kaplan-Meier analysis. D) Real-time analysis of mRNA levels of proinflammatory cytokines, including IL-1β and TNF-α, in the absence or presence of LPS in peritoneal macrophages; n = 3–4. Ctrl, control treatment. *P < 0.05 vs. ND LPS group; **P < 0.05 vs. ND control group. All values show means ±
sd
.
Similar articles
- Behavioral stress reduces RIP140 expression in astrocyte and increases brain lipid accumulation.
Feng X, Lin YL, Wei LN. Feng X, et al. Brain Behav Immun. 2015 May;46:270-9. doi: 10.1016/j.bbi.2015.02.008. Epub 2015 Feb 16. Brain Behav Immun. 2015. PMID: 25697398 Free PMC article. - MicroRNA-101 overexpression by IL-6 and TNF-α inhibits cholesterol efflux by suppressing ATP-binding cassette transporter A1 expression.
Zhang N, Lei J, Lei H, Ruan X, Liu Q, Chen Y, Huang W. Zhang N, et al. Exp Cell Res. 2015 Aug 1;336(1):33-42. doi: 10.1016/j.yexcr.2015.05.023. Epub 2015 May 29. Exp Cell Res. 2015. PMID: 26033364 - Regulation of the MIR155 host gene in physiological and pathological processes.
Elton TS, Selemon H, Elton SM, Parinandi NL. Elton TS, et al. Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review. - Biological activities of receptor-interacting protein 140 in adipocytes and metabolic diseases.
Ho PC, Wei LN. Ho PC, et al. Curr Diabetes Rev. 2012 Nov;8(6):452-7. doi: 10.2174/157339912803529922. Curr Diabetes Rev. 2012. PMID: 22934550 Free PMC article. Review.
Cited by
- M1-M2 balancing act in white adipose tissue browning - a new role for RIP140.
Liu PS, Lin YW, Burton FH, Wei LN. Liu PS, et al. Adipocyte. 2015 Jan 7;4(2):146-8. doi: 10.4161/21623945.2014.981428. eCollection 2015 Apr-Jun. Adipocyte. 2015. PMID: 26167418 Free PMC article. - miR-33 in cardiometabolic diseases: lessons learned from novel animal models and approaches.
Price NL, Goedeke L, Suárez Y, Fernández-Hernando C. Price NL, et al. EMBO Mol Med. 2021 May 7;13(5):e12606. doi: 10.15252/emmm.202012606. Epub 2021 May 3. EMBO Mol Med. 2021. PMID: 33938628 Free PMC article. Review. - Down-Regulated Receptor Interacting Protein 140 Is Involved in Lipopolysaccharide-Preconditioning-Induced Inactivation of Kupffer Cells and Attenuation of Hepatic Ischemia Reperfusion Injury.
Yuan G, Yu Y, Ji L, Jie X, Yue L, Kang Y, Jianping G, Zuojin L. Yuan G, et al. PLoS One. 2016 Oct 10;11(10):e0164217. doi: 10.1371/journal.pone.0164217. eCollection 2016. PLoS One. 2016. PMID: 27723769 Free PMC article. - Metabolic communication in tumors: a new layer of immunoregulation for immune evasion.
Ho PC, Liu PS. Ho PC, et al. J Immunother Cancer. 2016 Feb 16;4:4. doi: 10.1186/s40425-016-0109-1. eCollection 2016. J Immunother Cancer. 2016. PMID: 26885366 Free PMC article. Review. - MicroRNA modulation of lipid metabolism and oxidative stress in cardiometabolic diseases.
Aranda JF, Madrigal-Matute J, Rotllan N, Fernández-Hernando C. Aranda JF, et al. Free Radic Biol Med. 2013 Sep;64:31-9. doi: 10.1016/j.freeradbiomed.2013.07.014. Epub 2013 Jul 17. Free Radic Biol Med. 2013. PMID: 23871755 Free PMC article. Review.
References
- Maxfield F. R., Tabas I. (2005) Role of cholesterol and lipid organization in disease. Nature 438, 612–621 - PubMed
- Russell J. A. (2006) Management of sepsis. N. Engl. J. Med. 355, 1699–1713 - PubMed
- Vachharajani V., Vital S. (2006) Obesity and sepsis. J. Intens. Care Med. 21, 287–295 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK060521/DK/NIDDK NIH HHS/United States
- DK060521-07S1/DK/NIDDK NIH HHS/United States
- P50 DA011806/DA/NIDA NIH HHS/United States
- DA11190/DA/NIDA NIH HHS/United States
- DK60521/DK/NIDDK NIH HHS/United States
- K02 DA013926/DA/NIDA NIH HHS/United States
- R01 DA011190/DA/NIDA NIH HHS/United States
- R01 DK054733/DK/NIDDK NIH HHS/United States
- K02-DA13926/DA/NIDA NIH HHS/United States
- DK054733-09S1/DK/NIDDK NIH HHS/United States
- DK54733/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases