Linear ubiquitin assembly complex negatively regulates RIG-I- and TRIM25-mediated type I interferon induction - PubMed (original) (raw)
Linear ubiquitin assembly complex negatively regulates RIG-I- and TRIM25-mediated type I interferon induction
Kyung-Soo Inn et al. Mol Cell. 2011.
Abstract
Upon detection of viral RNA, retinoic acid-inducible gene I (RIG-I) undergoes TRIM25-mediated K63-linked ubiquitination, leading to type I interferon (IFN) production. In this study, we demonstrate that the linear ubiquitin assembly complex (LUBAC), comprised of two RING-IBR-RING (RBR)-containing E3 ligases, HOIL-1L and HOIP, independently targets TRIM25 and RIG-I to effectively suppress virus-induced IFN production. RBR E3 ligase domains of HOIL-1L and HOIP bind and induce proteasomal degradation of TRIM25, whereas the NZF domain of HOIL-1L competes with TRIM25 for RIG-I binding. Consequently, both actions by the HOIL-1L/HOIP LUBAC potently inhibit RIG-I ubiquitination and antiviral activity, but in a mechanistically separate manner. Conversely, the genetic deletion or depletion of HOIL-1L and HOIP robustly enhances virus-induced type I IFN production. Taken together, the HOIL-1L/HOIP LUBAC specifically suppresses RIG-I ubiquitination and activation by inducing TRIM25 degradation and inhibiting TRIM25 interaction with RIG-I, resulting in the comprehensive suppression of the IFN-mediated antiviral signaling pathway.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
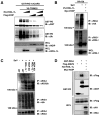
Figure 1. HOIL-1L/HOIP interacts with TRIM25 and RIG-I
(A–B) Interactions between TRIM25 and HOIL-1L or HOIP. 293T were transfected with V5-TRIM25 together with (A) HA-HOIL-1L or (B) Flag-HOIP, followed by co-IP and IB. (C) HOIL-1L interacts with RIG-I. 293T cells transfected with GST-RIG-2CARD and V5-HOIL-1L or vector were used for GST-pull down (PD) assay and IB. (D) HOIP requires HOIL-1L to interact with RIG-I. GST-RIG-2CARD and Flag-HOIP were transfected with increasing amounts of HOIL-1L, followed by IP with anti-Flag. (E) Endogenous HOIL-1L/HOIP interacts with RIG-I and TRIM25. WT MEFs were mock-infected or infected with SeV for 6 h and treated with MG132 for 4 h before harvest, followed by IP with control IgG, anti-RIG-I or anti-TRIM25. (F) Co-localization of HOIL-1L/HOIP with TRIM25. HeLa cells were mock-treated or infected with SeV for 10 h, followed by fixation and staining with anti-TRIM25, anti-HOIL-1L or anti-HOIP antibody. Co-localization between TRIM25 and HOIL-1L, Pearson’s correlation=0.871 and Mander’s overlap=0.885; co-localization between TRIM25 and HOIP, Pearson’s correlation=0.702 and Mander’s overlap=0.955 (G) TRIM25 SPRY domain is responsible for HOIL-1L/HOIP interaction. HA-HOIL-1L or Flag-HOIP was transfected with TRIM25 deletion mutants, followed by co-IP. (H) Binding ability of HOIL-1L or HOIP mutants. Deletion mutants of HOIL-1L or HOIP were transfected with TRIM25 or GST-RIG-I 2CARD as indicated. Cells were treated with MG132 for 6 h, followed by Co-IP and IB. (I) RIG-I interaction with HOIL-1L/HOIP in TRIM25−/− MEFs. WT and TRIM25−/− MEFs were infected with SeV for 10 h, followed by co-IP using control IgG or anti-RIG-I. (J) In vitro interaction between RIG-I-2CARD and HOIL-1L. Bacterially purified GST fusion proteins of RIG-I-2CARD, HOIL-1L ΔRBR-V5 and HOIP ΔRBR-Flag were used for in vitro binding, followed by co-IP and IB as indicated. See also Figure S1.
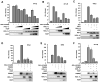
Figure 2. HOIL-1L/HOIP complex negatively regulates RIG-I mediated IFN-β signaling
(A–B) Inhibition of RIG-I-2CARD induced IFN-β and NF-κB promoter activities by HOIL-1L/HOIP. 293T cells were transfected with RIG-I-2CARD with increasing amounts of HOIL-1L, HOIP, or both together with IFN-β (A) or NF-κB promoter (B). (C) Inhibition of SeV induced IFN-β promoter activity by HOIL-1L/HOIP. IFN-β promoter activities were measured from 293T cells transfected with full-length RIG-I and HOIL-1L and/or HOIP. At 24 h after transfection, cells were mock-infected or infected with SeV (40 HAU/ml) for 10 h before luciferase assay. (D-F) RIG-I-2CARD, TRIM25, HOIL-1L and HOIP plasmids were transfected together with IFN-β (D), ISRE (E), or NF-κB (F) reporter plasmids into 293T. All luciferase assays were performed at least three times and graphs show the mean ± SD. Values are normalized by pRL-TK Renilla.
Figure 3. HOIL-1L/HOIP depletion increases IFN-β production and anti-viral response
(A) WT, HOIL-1L−/−, HOIP knock-down MEF (HOIP KD), HOIL-1L−/−-HOIP KD MEFs were established as described in the Experimental Procedures. Depletion and reduction of HOIL-1L and HOIP, respectively, were confirmed by IB. (B) Enhanced IFN-β promoter activity in HOIL-1L/HOIP depleted MEFs. MEFs were infected with SeV (50 HAU/ml) for 10 h and then subjected to dual-luciferase assay. Experiments were performed in triplicate and graph shows the mean ± SD. (C) Increased IFN-β production in HOIL-1L/HOIP depleted MEFs. MEFs were infected with SeV (50 HAU/ml) for 12 h and supernatants were subjected to mouse IFN-β ELISA. Experiments were performed in triplicate and graph shows the mean ± SD. (D) Suppression of IFN-β promoter activity in HOIL-1L−/− MEFs by complementation of HOIL-1L. HOIL-1L was co-transfected with reporter plasmid as indicated. Virus infection and luciferase assay were performed similarly to (A). Experiments were performed in triplicate and graph shows the mean ± SD. (E–F) Viral replication in HOIL-1L/HOIP depleted MEFs. MEFs were infected with VSV-eGFP (M.O.I=0.02). Supernatants were taken at 48 h p.i. and subjected to plaque assay. (G) VSV-eGFP replication in 293T expressing vector alone or HOIL-1L.
Figure 4. HOIL-1L/HOIP LUBAC inhibits TRIM25-mediated RIG-I ubiquitination
(A) Inhibition of RIG-I ubiquitination by HOIL-1L/HOIP. 293T cells transfected with GST-RIG-I-2CARD and TRIM25 together with HOIL-1L or HOIP were subjected to GST-PD and IB. (B) Inhibition of endogenous RIG-I ubiquitination by HOIL-1L/HOIP. 293T cells transfected with HA-ubiquitin with or without HOIL-1L/HOIP were mock-infected or infected with SeV for 10 h, followed by IP with anti-RIG-I. (C) RIG-I ubiquitination in HOIL-1L/HOIP depleted MEF. WT, HOIL-1L−/−, HOIP-KD and HOIL-1L−/−-HOIP-KD MEFs infected with SeV were used for IP and IB. Anti-ubiquitin (αUb) antibody and anti-K63-ubiquitin chain specific antibody (αK63) were used to detect RIG-I ubiquitination. (D) Decreased interaction between RIG-I and MAVS by HOIL-1L/HOIP expression. 293T cells transfected with Flag-RIG-I-2CARD, GST-MAVS-CARD-proline-rich domain (PRD), V5-HOIL-1L and/or Myc-HOIP were used for co-IP and IB. See also Figure S2.
Figure 5. HOIL-1L/HOIP LUBAC induces TRIM25 ubiquitination and degradation
(A) Increment of TRIM25 levels in HOIL-1L/HOIP depleted MEFs. WT, HOIL-1L−/−, HOIP KD, HOIL-1L−/−-HOIP KD MEFs were mock-infected or infected with SeV for 12 h. Cell lysates were subjected to IB. (B) Decreased endogenous TRIM25 levels upon HOIL-1L/HOIP overexpression. Endogenous TRIM25 levels were analyzed by IB and densitometry. IBs show representative data, and graph shows the averages of triplicate. (C) Reduction of TRIM25 by HOIL-1L/HOIP expression. At 36 h after the transfection with V5-TRIM25 and GST together with HOIL-1L and HOIP as indicated, 293T cells were mock-treated or treated with MG132 for 6 h and were subjected to IB. (D) TRIM25 ubiquitination induced by HOIL-1L/HOIP. 293T cells were transfected with V5-TRIM25, HOIL-1L and HOIP and MG132 treatment for IP. (E) Endogenous TRIM25 ubiquitination upon SeV infection. MEFs were mock-infected or infected with SeV for 10 h and cell lysates were subjected to IP using anti-TRIM25 antibody. (F) TRIM25 ubiquitination levels in HOIL-1L/HOIP depleted MEFs. MEFs were infected with SeV and treated with MG132, followed by IP with anti-TRIM25 and IB with anti-Ub or anti-TRIM25. (G) TRIM25 ubiquitination by HOIL-1L/HOIP RINGCS mutants. 293T cells were transfected with V5-TRIM25 and WT or RINGCS mutants of HOIL-1L or HOIP, followed by IP with anti-V5 and IB with anti-Ub or anti-V5. (H) In vitro ubiquitination of TRIM25 by LUBAC. Purified GST-TRIM25 RINGCS was subjected to an in vitro ubiquitination with baculovirus-purified HOIL-1L/HOIP LUBAC, followed by IB with anti-GST antibody. See also Figure S3.
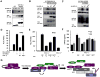
Figure 6. HOIL-1L/HOIP LUBAC inhibits the interaction between RIG-I and TRIM25
(A) Inhibition of RIG-I ubiquitination by HOIL-1L/HOIP RINGCS mutants. 293T cells were transfected with GST-RIG-I-2CARD and TRIM25 together with HOIL-1L, HOIP, or RINGCS mutants and treated with MG132, followed by GST-PD. (B) Inhibition of RIG-I-2CARD and TRIM25 interaction by HOIL-1L/HOIP. 293T cells were transfected with GST-RIG-I-2CARD and TRIM25 together with HOIL-1L and/or HOIP as indicated, followed by GST-PD. (C) Increased interaction between RIG-I and TRIM25 in HOIL-1L/HOIP depleted MEFs. MEFs were mock-infected or infected with SeV (50 HAU/ml) for 12 h and subjected to co-IP using control IgG or anti-RIG-I. (D) Time-course analysis of endogenous RIG-I and TRIM25 interaction. At different time points after SeV infection, the same amounts of proteins from WT MEFs were subjected to co-IP using anti-TRIM25. (E) In vitro competition assay. Bacterially purified GST-RIG-I-2CARD and GST-TRIM25 were incubated with increasing amounts of GST-HOIL-1L ΔRBR-V5 or GST-HOIP ΔRBR-Flag, followed by IP with anti-RIG-I. (F) Inhibition of RIG-I and TRIM25 interaction by LUBAC. Biotin-labeled GST-TRIM25 were added to GST-RIG-I coated wells with increasing amounts of GST-HOIL-1L ΔRBR-V5/GST-HOIP ΔRBR-Flag (LUBAC) or unlabeled GST-TRIM25. Bound biotin-labeled GST-TRIM25 was detected using streptavidin-HRP. Experiments were performed in triplicate and graph shows the mean ± SD. See also Figure S4.
Figure 7. Roles of the NZF and RBR domains of HOIL-1L in RIG-I mediated IFN-β signaling
(A) Inability of HOIL-1L NZFCS mutant to bind RIG-I. 293T cells were transfected with GST-RIG-I-2CARD together with HA-HOIL-1L WT or NZFCS mutant, followed by GST-PD and IB with indicated antibodies. (B) Inability of HOIL-1L NZFCS mutant to interfere the RIG-I-TRIM25 interaction. 293T cells were transfected with GST-RIG-I-2CARD and TRIM25 together with HA-HOIL-1L WT or NZFCS mutants and treated with MG132, followed by GST-PD and IB. (C) Inability of HOIL-1L NZFCS mutant to inhibit RIG-I ubiquitination. 293T cells were transfected with GST-RIG-I-2CARD and TRIM25 together with HOIL-1L WT or NZFCS mutant as indicated, followed by GST-PD and IB. (D) Effect of HOIL-1L WT or NZFCS mutant on RIG-I-mediated IFN-β promoter activity. IFN-β promoter activities were determined from 293T cells transfected with RIG-I-2CARD, and TRIM25 together with HOIL-1L WT or NZFCS mutant. Experiments were performed in triplicate and graph shows the mean ± SD. (E-F) Complementation of HOIL-1L−/− MEFs. HOIL-1L−/− MEFs were infected with recombinant retrovirus containing HOIL-1L WT, RINGCS, or NZFCS mutant, followed by the puromycin antibiotic selection. (E) Complemented MEFs were transfected with IFN-β reporter, followed by mock-infection or SeV infection and luciferase assay. Experiments were performed in triplicate and graph shows the mean ± SD. (F) Complemented MEFs were mock-infected or infected with SeV (50 HAU/ml) and supernatants were subjected to IFN-β ELISA. Experiments were performed in triplicate and graph shows the mean ± SD. (G) Hypothetical model for LUBAC-mediated inhibition of RIG-I and TRIM25 pathway. See also Figure S5.
Similar articles
- The ubiquitin-specific protease USP15 promotes RIG-I-mediated antiviral signaling by deubiquitylating TRIM25.
Pauli EK, Chan YK, Davis ME, Gableske S, Wang MK, Feister KF, Gack MU. Pauli EK, et al. Sci Signal. 2014 Jan 7;7(307):ra3. doi: 10.1126/scisignal.2004577. Sci Signal. 2014. PMID: 24399297 Free PMC article. - Activation of duck RIG-I by TRIM25 is independent of anchored ubiquitin.
Miranzo-Navarro D, Magor KE. Miranzo-Navarro D, et al. PLoS One. 2014 Jan 23;9(1):e86968. doi: 10.1371/journal.pone.0086968. eCollection 2014. PLoS One. 2014. PMID: 24466302 Free PMC article. - Mechanistic insights into the subversion of the linear ubiquitin chain assembly complex by the E3 ligase IpaH1.4 of Shigella flexneri.
Liu J, Wang Y, Wang D, Wang Y, Xu X, Zhang Y, Li Y, Zhang M, Gong X, Tang Y, Shen L, Li M, Pan L. Liu J, et al. Proc Natl Acad Sci U S A. 2022 Mar 22;119(12):e2116776119. doi: 10.1073/pnas.2116776119. Epub 2022 Mar 16. Proc Natl Acad Sci U S A. 2022. PMID: 35294289 Free PMC article. - Ubiquitin-mediated modulation of the cytoplasmic viral RNA sensor RIG-I.
Oshiumi H, Matsumoto M, Seya T. Oshiumi H, et al. J Biochem. 2012 Jan;151(1):5-11. doi: 10.1093/jb/mvr111. Epub 2011 Sep 2. J Biochem. 2012. PMID: 21890623 Review. - Linear Ubiquitin Code: Its Writer, Erasers, Decoders, Inhibitors, and Implications in Disorders.
Oikawa D, Sato Y, Ito H, Tokunaga F. Oikawa D, et al. Int J Mol Sci. 2020 May 11;21(9):3381. doi: 10.3390/ijms21093381. Int J Mol Sci. 2020. PMID: 32403254 Free PMC article. Review.
Cited by
- Hepatitis B Virus-Induced Parkin-Dependent Recruitment of Linear Ubiquitin Assembly Complex (LUBAC) to Mitochondria and Attenuation of Innate Immunity.
Khan M, Syed GH, Kim SJ, Siddiqui A. Khan M, et al. PLoS Pathog. 2016 Jun 27;12(6):e1005693. doi: 10.1371/journal.ppat.1005693. eCollection 2016 Jun. PLoS Pathog. 2016. PMID: 27348524 Free PMC article. - Multilayered regulations of RIG-I in the anti-viral signaling pathway.
Kim N, Now H, Nguyen NTH, Yoo JY. Kim N, et al. J Microbiol. 2016 Sep;54(9):583-587. doi: 10.1007/s12275-016-6322-2. Epub 2016 Aug 31. J Microbiol. 2016. PMID: 27572506 Review. - HOIL1 Is Essential for the Induction of Type I and III Interferons by MDA5 and Regulates Persistent Murine Norovirus Infection.
MacDuff DA, Baldridge MT, Qaqish AM, Nice TJ, Darbandi AD, Hartley VL, Peterson ST, Miner JJ, Iwai K, Virgin HW. MacDuff DA, et al. J Virol. 2018 Nov 12;92(23):e01368-18. doi: 10.1128/JVI.01368-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30209176 Free PMC article. - Molecular control of the NEMO family of ubiquitin-binding proteins.
Clark K, Nanda S, Cohen P. Clark K, et al. Nat Rev Mol Cell Biol. 2013 Oct;14(10):673-85. doi: 10.1038/nrm3644. Epub 2013 Aug 29. Nat Rev Mol Cell Biol. 2013. PMID: 23989959 Review. - Trim25 Is an RNA-Specific Activator of Lin28a/TuT4-Mediated Uridylation.
Choudhury NR, Nowak JS, Zuo J, Rappsilber J, Spoel SH, Michlewski G. Choudhury NR, et al. Cell Rep. 2014 Nov 20;9(4):1265-72. doi: 10.1016/j.celrep.2014.10.017. Cell Rep. 2014. PMID: 25457611 Free PMC article.
References
- Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19 AI083025-02S1/AI/NIAID NIH HHS/United States
- R37 AI087846/AI/NIAID NIH HHS/United States
- AI083355/AI/NIAID NIH HHS/United States
- RR00168/RR/NCRR NIH HHS/United States
- AI083025/AI/NIAID NIH HHS/United States
- R01 CA082057/CA/NCI NIH HHS/United States
- U19 AI083025/AI/NIAID NIH HHS/United States
- CA082057/CA/NCI NIH HHS/United States
- U19 AI083025-03/AI/NIAID NIH HHS/United States
- P51 RR000168/RR/NCRR NIH HHS/United States
- K26 RR000168/RR/NCRR NIH HHS/United States
- R01 AI073099-05/AI/NIAID NIH HHS/United States
- AI087846-01A1/AI/NIAID NIH HHS/United States
- R01 AI087846/AI/NIAID NIH HHS/United States
- R01 AI073099/AI/NIAID NIH HHS/United States
- R01 AI083355-03/AI/NIAID NIH HHS/United States
- R01 AI083355/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases