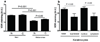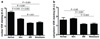Expression of vitamin D receptor decreases during progression of pigmented skin lesions - PubMed (original) (raw)
Expression of vitamin D receptor decreases during progression of pigmented skin lesions
Anna A Brożyna et al. Hum Pathol. 2011 May.
Abstract
1,25-dihydroxyvitamin D3 affects proliferation, differentiation, and apoptosis and protects DNA against oxidative damage with a net tumorostatic and anticarcinogenic effect. It acts through a specific nuclear receptor that is widely distributed through the body. Although a beneficial role of vitamin D in melanoma patients has been suggested, there is lack of information on the changes in the expression pattern of vitamin D receptor during progression of pigmented lesions. Using immunohistochemistry, we analyzed the expression of vitamin D receptor in 140 samples obtained form 82 patients, including 25 benign nevi, 70 primary cutaneous melanomas, 35 metastases, 5 re-excisions, and 5 normal skin biopsies. The strongest expression was observed in normal skin that significantly decreased in melanocytic proliferations with the following order of expression: normal skin > melanocytic nevi > melanomas = metastases. The vitamin D receptor expression in skin surrounding nevi and melanoma was also significantly reduced as compared to normal skin. Tumor-infiltrating and lymph node lymphocytes retained high levels of vitamin D receptor. There was negative correlation between tumor progression and vitamin D receptor expression with a remarkable decrease of the immunoreactivity in nuclei of melanoma cells at vertical versus radial growth phases and with metastatic melanomas showing the lowest cytoplasmic receptor staining. Furthermore, lack of the receptor expression in primary melanomas and metastases was related to shorter overall patients' survival. In addition, the receptor expression decreased in melanized melanoma cells in comparison to amelanotic or poorly pigmented cells. Therefore, we propose that reduction or absence of vitamin D receptor is linked to progression of melanocytic lesions, that its lack affects survival of melanoma patients, and that melanogenesis can attenuate receptor expression. In conclusion, changes in vitamin D receptor expression pattern can serve as important variables for diagnosis, predicting clinical outcome of the disease, and/or as a guidance for novel therapy of melanomas based on use of vitamin D or its derivatives.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Figure 1
Localization of VDR in primary skin pigmented lesions and metastases of melanoma. Compound (a), dermal (b), desmoplastic (c) nevi revealed stronger VDR staining (pink-red) than radial (d) and vertical (e) growing cells of SSM and radial (f) and vertical (g) growing cells of NMM. Lymphocytes infiltrating tumor (h) and lymph nodes lymphocytes (i) also revealed stronger VDR expression than melanoma cells. Melanoma arising (j) in nevus (k) showed weaker VDR expression. Nevus section used as negative control (j). Arrows indicate melanoma cells, double arrows indicate nevi cells, asterisks indicate melanin (brown), arrow heads indicate lymphocytes. Square indicates tumor-infiltrating lymphocytes enlarged in insert. Scale bar: 50 µm.
Figure 2
Supracellular and intraepidermal localization of VDR immunoreactivity. a. VDR immunoreactivity in skin pigmented lesions and melanomas metastases was seen in cell cytoplasm and nuclei. N-nuclear VDR staining, C-cytoplasmic VDR staining. b. VDR immunoreactivity in normal skin keratinocytes. A.U. – arbitrary units.
Figure 3
VDR expression in human skin pigmented lesions. During development of skin pigmented lesions VDR immunostaining decreased both in nuclei (a) and cytoplasm (b) of the cells. Normal-normal skin, MM-primary melanomas. A.U. – arbitrary units.
Figure 4
VDR expression changes during progression of melanocytic lesions and is determined by histological type and aggressiveness of melanomas. a. VDR nuclear staining in nevi and melanomas assessed according to Clark’s level. b. VDR cytoplasmic staining in nevi and melanomas assessed according Clark’s level. c. VDR nuclear staining in nevi and melanomas assessed according Breslow’s thickness. d. VDR cytoplasmic staining in nevi and melanomas assessed according Breslow’s thickness. e. VDR immunoreactivity in nuclei of radial and vertical growth phases of nodular (NMM) and superficial spreading (SSM) melanomas. f. VDR immunoreactivity in cytoplasm of radial and vertical growth phases of nodular (NMM) and superficial spreading (SSM) melanomas. g. Comparison of VDR expression in nodular (NMM) and superficial spreading (SSM) melanomas. h. Comparison of VDR expression in nuclei of normal skin and primary metastasing and non-metastasing melanomas. i. Comparison of VDR expression in cytoplasm of normal skin and primary metastasing and non-metastasing melanomas. A.U. – arbitrary units.
Figure 5
Type of pigmented lesion affects VDR expression in epidermal keratinocytes. Comparison of VDR expression in basal (a) and suprabasal (b) keratinocytes surrounding tumor. Representative VDR immunocytochemistry in normal skin (c) and skin adjacent to melanoma (d). Arrows point to cytoplasmic VDR staining; arrowheads point to nuclear VDR staining, D: dermis, SB: suprabasal keratinocytes; B: basal keratinocytes; scale bar: 50 µm. A.U. – arbitrary units.
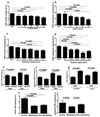
Figure 6
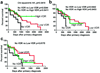
The dependence of overall survival time (OS) on VDR expression in nuclei of radial growth phase (a) and cytoplasm of vetrical growi phase (b) of primary melanomas and in nuclei of metastases (c). Data were analyzed using log-rank (Mantel-Cox) test.
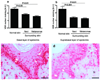
Figure 7
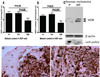
VDR expression is inversely correlated to melanin synthesis in melanoma cells. a. Comparison of VDR expression in radial growth phase. b. Comparison of VDR expression in vertical growth phase. c. WB analysis shows decreased expression of VDR in pigmented cells (P) in comparison to non-pigmented cells (NP). Beta-actin stain shows the equal loading of the protein. The relative melanization of cells is visualized in cell pellets on the bottom of the panel. d. Illustration of lack or low expression of VDR at the radial growth phase e. Illustration of lack or low expression of VDR at the vertical growth phase. Arrows point to cytoplasm, arrowheads point to nuclei; E: epidermis; Scale bar: 50 µm. A.U. – arbitrary units.
Similar articles
- Expression of the vitamin D-activating enzyme 1α-hydroxylase (CYP27B1) decreases during melanoma progression.
Brożyna AA, Jóźwicki W, Janjetovic Z, Slominski AT. Brożyna AA, et al. Hum Pathol. 2013 Mar;44(3):374-87. doi: 10.1016/j.humpath.2012.03.031. Epub 2012 Sep 17. Hum Pathol. 2013. PMID: 22995334 Free PMC article. - Beclin 1 and LC3 autophagic gene expression in cutaneous melanocytic lesions.
Miracco C, Cevenini G, Franchi A, Luzi P, Cosci E, Mourmouras V, Monciatti I, Mannucci S, Biagioli M, Toscano M, Moretti D, Lio R, Massi D. Miracco C, et al. Hum Pathol. 2010 Apr;41(4):503-12. doi: 10.1016/j.humpath.2009.09.004. Epub 2009 Dec 11. Hum Pathol. 2010. PMID: 20004946 - Cadherin expression pattern in melanocytic tumors more likely depends on the melanocyte environment than on tumor cell progression.
Krengel S, Grotelüschen F, Bartsch S, Tronnier M. Krengel S, et al. J Cutan Pathol. 2004 Jan;31(1):1-7. doi: 10.1046/j.0303-6987.2004.0106.x. J Cutan Pathol. 2004. PMID: 14675278 - Role of In Vivo Reflectance Confocal Microscopy in the Analysis of Melanocytic Lesions.
Serban ED, Farnetani F, Pellacani G, Constantin MM. Serban ED, et al. Acta Dermatovenerol Croat. 2018 Apr;26(1):64-67. Acta Dermatovenerol Croat. 2018. PMID: 29782304 Review. - Black and Brown Oro-facial Mucocutaneous Neoplasms.
Natarajan E. Natarajan E. Head Neck Pathol. 2019 Mar;13(1):56-70. doi: 10.1007/s12105-019-01008-2. Epub 2019 Jan 29. Head Neck Pathol. 2019. PMID: 30693458 Free PMC article. Review.
Cited by
- The Role of Classical and Novel Forms of Vitamin D in the Pathogenesis and Progression of Nonmelanoma Skin Cancers.
Slominski AT, Brożyna AA, Zmijewski MA, Janjetovic Z, Kim TK, Slominski RM, Tuckey RC, Mason RS, Jetten AM, Guroji P, Reichrath J, Elmets C, Athar M. Slominski AT, et al. Adv Exp Med Biol. 2020;1268:257-283. doi: 10.1007/978-3-030-46227-7_13. Adv Exp Med Biol. 2020. PMID: 32918223 Free PMC article. Review. - Novel vitamin D analogs as potential therapeutics: metabolism, toxicity profiling, and antiproliferative activity.
Chen J, Wang J, Kim TK, Tieu EW, Tang EK, Lin Z, Kovacic D, Miller DD, Postlethwaite A, Tuckey RC, Slominski AT, Li W. Chen J, et al. Anticancer Res. 2014 May;34(5):2153-63. Anticancer Res. 2014. PMID: 24778017 Free PMC article. - The Impact of Vitamin D on Skin Aging.
Bocheva G, Slominski RM, Slominski AT. Bocheva G, et al. Int J Mol Sci. 2021 Aug 23;22(16):9097. doi: 10.3390/ijms22169097. Int J Mol Sci. 2021. PMID: 34445803 Free PMC article. Review. - Participation of keratinocyte- and fibroblast-derived factors in melanocyte homeostasis, the response to UV, and pigmentary disorders.
Upadhyay PR, Ho T, Abdel-Malek ZA. Upadhyay PR, et al. Pigment Cell Melanoma Res. 2021 Jul;34(4):762-776. doi: 10.1111/pcmr.12985. Epub 2021 May 24. Pigment Cell Melanoma Res. 2021. PMID: 33973367 Free PMC article. Review. - Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes.
Slominski AT, Janjetovic Z, Kim TK, Wright AC, Grese LN, Riney SJ, Nguyen MN, Tuckey RC. Slominski AT, et al. Anticancer Res. 2012 Sep;32(9):3733-42. Anticancer Res. 2012. PMID: 22993313 Free PMC article.
References
- Holick M, MacLaughlin JA, Clark MB, Holick SA, Potts JT, Anderson RR, Blank IH, Parrish JA, Elias P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science. 1980;210:203–205. - PubMed
- Holick M. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88:296–307. - PubMed
- Lehmann B. Role of the vitamin D3 pathway in healthy and diseased skin-facts, contradictions and hypotheses. Exp Dermatol. 2009;18:97–108. - PubMed
- Kramer C, Seltmann H, Seifert M, Tilgen W, Zouboulis CC, Reichrath J. Characterization of the vitamin D endocrine system in human sebocytes in vitro. J Steroid Biochem Mol Biol. 2009;113:9–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR052190/AR/NIAMS NIH HHS/United States
- R01 AR052190-04/AR/NIAMS NIH HHS/United States
- R01 AR052190-05/AR/NIAMS NIH HHS/United States
- AR052190/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical