Autophagy failure in Alzheimer's disease--locating the primary defect - PubMed (original) (raw)
Review
Autophagy failure in Alzheimer's disease--locating the primary defect
Ralph A Nixon et al. Neurobiol Dis. 2011 Jul.
Abstract
Autophagy, the major degradative pathway for organelles and long-lived proteins, is essential for the survival of neurons. Mounting evidence has implicated defective autophagy in the pathogenesis of several major neurodegenerative diseases, particularly Alzheimer's disease (AD). A continuum of abnormalities of the lysosomal system has been identified in neurons of the AD brain, including pathological endocytic pathway responses at the very earliest disease stage and a progressive disruption of autophagy leading to the massive buildup of incompletely digested substrates within dystrophic axons and dendrites. In this review, we examine research on autophagy in AD and evaluate evidence addressing the specific step or steps along the autophagy pathway that may be defective. Current evidence strongly points to disruption of substrate proteolysis within autolysosomes for the principal mechanism underlying autophagy failure in AD. In the most common form of familial early onset AD, mutant presenilin 1 disrupts autophagy directly by impeding lysosomal proteolysis while, in other forms of AD, autophagy impairments may involve different genetic or environmental factors. Attempts to restore more normal lysosomal proteolysis and autophagy efficiency in mouse models of AD pathology have yielded promising therapeutic effects on neuronal function and cognitive performance, demonstrating the relevance of autophagy failure to the pathogenesis of AD and the potential of autophagy modulation as a therapeutic strategy. This article is part of a Special Issue entitled "Autophagy and protein degradation in neurological diseases."
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
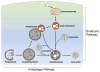
Figure 1
Schematic illustrating the endocytic and the autophagic pathways to the lysosome. Internalized materials entering the endocytic pathway are directed to early endosomes, which mature to late endosomes/multivesicular bodies. In the autophagic pathway, a phagophore sequesters an area of cytoplasm containing organelles to form a double membrane-limited autophagosome. The formation of amphisomes via the fusion between autophagosomes and late endosomes/multivesicular bodies provides an interactive point between the two pathways. Autophagosomes, and amphisomes in the two pathways receive hydrolases by fusing with lysosomes to form autolysosomes or late endosomes/multivesicular bodies to form amphisomes. Efficient digestion of substrates within these compartments yields lysosomes containing mainly acid hydrolases.
Figure 2
Autophagy homeostasis requires a delicate balance between autophagosome formation and clearance. Despite a constitutive level of autophagic protein turnover, normal cells in their basal state display dense lysosomes (arrow) but relatively few autophagic vacuoles (A). Strong autophagy induction, in this case by acute serum deprivation, elicits the robust formation of autophagic vacuoles (AVs)(arrows) because autophagosome formation now exceeds the rate of AV clearance (B). The loss of the capacity to properly acidify lysosomes, as it exists in the blastocyst-lacking presenilin 1, markedly slows lysosomal proteolysis and AV clearance without altering autophagy induction (Lee et al., 2010), and AVs (arrows) robustly accumulate (C). Although both experimental conditions induce AV accumulation, albeit with somewhat different composition, the rate of autophagic protein turnover is much higher than normal in the serum-deprived cells and much lower than normal in the PS1 KO cells.
Figure 3
The “burden” of AVs containing incompletely digested proteins stored in dystrophic neurites of the AD brain is great. At the light microscopic levels, CatD immunostaining in the AD brain (B) but not in the control brain (A) reveals a high density of senile plaques (B, arrows) each containing dozens of dystrophic neurites visible after histochemical staining for acid phosphatase (C, arrows) or by CatD immunocytochemistry (D). (E) Ultrastructural analysis of dystrophic neurites (circled by arrowheads) demonstrates that dystrophic neurites predominantly contain hundreds of AVs of distinct subtypes but the majority have double membranes with electron dense lumens, most consistent with autolysosomes and amphisomes in which proteolysis of substrates, including inner membrane, has stalled. Given the number of dystrophic neurites in the AD brain, the amount of “stored” wasted proteins is enormous, rivaling that in certain primary lysosomal storage disorders.
Similar articles
- Autophagy failure in Alzheimer's disease and the role of defective lysosomal acidification.
Wolfe DM, Lee JH, Kumar A, Lee S, Orenstein SJ, Nixon RA. Wolfe DM, et al. Eur J Neurosci. 2013 Jun;37(12):1949-61. doi: 10.1111/ejn.12169. Eur J Neurosci. 2013. PMID: 23773064 Free PMC article. - Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer's-like axonal dystrophy.
Lee S, Sato Y, Nixon RA. Lee S, et al. J Neurosci. 2011 May 25;31(21):7817-30. doi: 10.1523/JNEUROSCI.6412-10.2011. J Neurosci. 2011. PMID: 21613495 Free PMC article. - Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease.
Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA. Boland B, et al. J Neurosci. 2008 Jul 2;28(27):6926-37. doi: 10.1523/JNEUROSCI.0800-08.2008. J Neurosci. 2008. PMID: 18596167 Free PMC article. - Autophagy-lysosomal-associated neuronal death in neurodegenerative disease.
Nixon RA. Nixon RA. Acta Neuropathol. 2024 Sep 11;148(1):42. doi: 10.1007/s00401-024-02799-7. Acta Neuropathol. 2024. PMID: 39259382 Review. - Dysfunctional autophagy in Alzheimer's disease: pathogenic roles and therapeutic implications.
Liang JH, Jia JP. Liang JH, et al. Neurosci Bull. 2014 Apr;30(2):308-16. doi: 10.1007/s12264-013-1418-8. Epub 2014 Mar 7. Neurosci Bull. 2014. PMID: 24610177 Free PMC article. Review.
Cited by
- Bridging the Gap Between Fluid Biomarkers for Alzheimer's Disease, Model Systems, and Patients.
Bjorkli C, Sandvig A, Sandvig I. Bjorkli C, et al. Front Aging Neurosci. 2020 Sep 2;12:272. doi: 10.3389/fnagi.2020.00272. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32982716 Free PMC article. Review. - Rapamycin prevents the mutant huntingtin-suppressed GLT-1 expression in cultured astrocytes.
Chen LL, Wu JC, Wang LH, Wang J, Qin ZH, Difiglia M, Lin F. Chen LL, et al. Acta Pharmacol Sin. 2012 Mar;33(3):385-92. doi: 10.1038/aps.2011.162. Epub 2012 Jan 23. Acta Pharmacol Sin. 2012. PMID: 22266730 Free PMC article. - Carnosic Acid Prevents Beta-Amyloid-Induced Injury in Human Neuroblastoma SH-SY5Y Cells via the Induction of Autophagy.
Liu J, Su H, Qu QM. Liu J, et al. Neurochem Res. 2016 Sep;41(9):2311-23. doi: 10.1007/s11064-016-1945-6. Epub 2016 May 11. Neurochem Res. 2016. PMID: 27168327 - Lipopolysaccharide-induced Autophagy Increases SOX2-positive Astrocytes While Decreasing Neuronal Differentiation in the Adult Hippocampus.
Liu WC, Wu CW, Fu MH, Tain YL, Liang CK, Chen IC, Hung CY, Lee YC, Wu KLH. Liu WC, et al. Exp Neurobiol. 2022 Oct 31;31(5):307-323. doi: 10.5607/en22005. Exp Neurobiol. 2022. PMID: 36351841 Free PMC article. - Inflammaging: disturbed interplay between autophagy and inflammasomes.
Salminen A, Kaarniranta K, Kauppinen A. Salminen A, et al. Aging (Albany NY). 2012 Mar;4(3):166-75. doi: 10.18632/aging.100444. Aging (Albany NY). 2012. PMID: 22411934 Free PMC article.
References
- Anglade P, et al. Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease. Histol Histopathol. 1997;12:25–31. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical