MRE11 deficiency increases sensitivity to poly(ADP-ribose) polymerase inhibition in microsatellite unstable colorectal cancers - PubMed (original) (raw)
MRE11 deficiency increases sensitivity to poly(ADP-ribose) polymerase inhibition in microsatellite unstable colorectal cancers
Eduardo Vilar et al. Cancer Res. 2011.
Abstract
Microsatellite instability (MSI) is displayed by approximately 15% of colorectal cancers (CRC). Defective DNA mismatch repair generates mutations at repetitive DNA sequences such as those located in the double strand break (DSB) repair gene MRE11. We assessed the mutational status of MRE11 in a panel of 17 CRC cell lines and 46 primary tumors and found a strong correlation with MSI status in both cell lines and tumors. Therefore, we hypothesized that deficiency in MRE11 may sensitize CRC cells to poly(ADP-ribose) polymerase (PARP-1) inhibition based on the concept of synthetic lethality. We further assessed the activity of the PARP-1 inhibitor, ABT-888, in CRC cell lines and observed preferential cytotoxicity in those MSI cell lines harboring mutations in MRE11 compared with both wild-type cell lines and microsatellite stable (MSS) cell lines. A significant correlation between MRE11 expression levels and cytotoxicity to ABT-888 at 10 μM was observed (R² = 0.915, P < 0.001). Using two experimental approaches, including short hairpin RNA knocking down MRE11 in the wild-type and MSS cell line SW-480 and a second cell line model transfected with mutant MRE11, we experimentally tried to confirm the role of MRE11 in conferring sensitivity to PARP-1 inhibition. Both models led to changes in proliferation in response to ABT-888 at different concentrations, and a drug-response effect was not observed, suggesting a possible contribution of additional genes. We conclude that MSI colorectal tumors deficient in DSB repair secondary to mutation in MRE11 show a higher sensitivity to PARP-1 inhibition. Further clinical investigation of PARP-1 inhibitors is warranted in MSI CRCs.
Figures
Figure 1
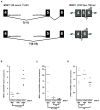
A) a polyT(11) tract located at the Intron4 is the target for mutations in MSI tumors. Shortening in one or more nucleotides causes changes in the splicing can induce complete skipping of exon 5 and protein truncation. Two sets of primers were designed to measure the levels of the wild type (wt) and the mutant transcript (mut) of MRE11; B), C) and D) levels of expression of the wt and mut transcript of MRE11, as well as PARP-1 assessed by qRT-PCR in 10 CRC cell lines.
Figure 2
Schematic representation of the homologous recombination pathway hsa03440 retrieved from the KEGG database. In red are those genes upregulated and in blue those downregulated. Note that not all of the proteins acting in this pathway are shown in this figure; B) Probe sets from hsa03440 significantly deregulated in MSI compared to MSS tumors and their corresponding fold-changes;
Figure 3
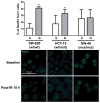
Rad51 foci were analyzed under baseline conditions (B, t=0) and 18 hours after irradiation (IR, t=18). Percentages of foci-positive cells at each time point are presented. Error bars represent standard errors of the mean for two independent observations. Asterisks designate statistically significant differences in percent positivity at baseline vs. 18 hours post-irradiation (_P-_value<0.05). Representative images are presented for both markers and time points.
Figure 4
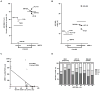
A) and B) Comparison of cytotoxicity at 10 μM and IC50 in biallelic mutants and wild-type plus monoallelic mutants; C) Correlation between expression levels of the MRE11 mutant transcript and growth inhibition at 10 μM of ABT-888; D) Cell cycle changes after treatment with two different concentrations of ABT-888. Note that cell cycle changes were more pronounced in biallelic than monoallelic mutants and wild type cells.
Figure 5
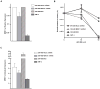
A) Levels of MRE11 wild type transcript in transfected cells with a shRNA plasmid against MRE11 and in the derivative cell line SM1.3 that has been transfected with an expression construct for Δ5-7MRE11 lacking exons 5–7. Levels of expression were normalized to mock cells and to the parental cell line SW480/SN3, respectively. B) Proliferation after treatment with ABT-888. Note a difference in proliferation between SW-480 transfected with shRNA anti-MRE11 plasmid and SW-480 transfected with a mock plasmid at 50 μM and also between SM1.3 and its parental cell line at 10 μM. C) Expression levels of the wild type transcript of MRE11 compared to levels expressed in the original panel of cell lines.
Similar articles
- Microsatellite instability induced mutations in DNA repair genes CtIP and MRE11 confer hypersensitivity to poly (ADP-ribose) polymerase inhibitors in myeloid malignancies.
Gaymes TJ, Mohamedali AM, Patterson M, Matto N, Smith A, Kulasekararaj A, Chelliah R, Curtin N, Farzaneh F, Shall S, Mufti GJ. Gaymes TJ, et al. Haematologica. 2013 Sep;98(9):1397-406. doi: 10.3324/haematol.2012.079251. Epub 2013 Jan 24. Haematologica. 2013. PMID: 23349304 Free PMC article. - Microsatellite instability due to hMLH1 deficiency is associated with increased cytotoxicity to irinotecan in human colorectal cancer cell lines.
Vilar E, Scaltriti M, Balmaña J, Saura C, Guzman M, Arribas J, Baselga J, Tabernero J. Vilar E, et al. Br J Cancer. 2008 Nov 18;99(10):1607-12. doi: 10.1038/sj.bjc.6604691. Epub 2008 Oct 21. Br J Cancer. 2008. PMID: 18941461 Free PMC article. - Evaluation of candidate biomarkers to predict cancer cell sensitivity or resistance to PARP-1 inhibitor treatment.
Oplustilova L, Wolanin K, Mistrik M, Korinkova G, Simkova D, Bouchal J, Lenobel R, Bartkova J, Lau A, O'Connor MJ, Lukas J, Bartek J. Oplustilova L, et al. Cell Cycle. 2012 Oct 15;11(20):3837-50. doi: 10.4161/cc.22026. Epub 2012 Sep 14. Cell Cycle. 2012. PMID: 22983061 Free PMC article. - [From poly(ADP-ribose) discovery to PARP inhibitors in cancer therapy].
Schreiber V, Illuzzi G, Héberlé E, Dantzer F. Schreiber V, et al. Bull Cancer. 2015 Oct;102(10):863-73. doi: 10.1016/j.bulcan.2015.07.012. Epub 2015 Sep 15. Bull Cancer. 2015. PMID: 26384693 Review. French. - Poly(ADP-ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders.
Henning RJ, Bourgeois M, Harbison RD. Henning RJ, et al. Cardiovasc Toxicol. 2018 Dec;18(6):493-506. doi: 10.1007/s12012-018-9462-2. Cardiovasc Toxicol. 2018. PMID: 29968072 Review.
Cited by
- Treatment with the PARP inhibitor, niraparib, sensitizes colorectal cancer cell lines to irinotecan regardless of MSI/MSS status.
Genther Williams SM, Kuznicki AM, Andrade P, Dolinski BM, Elbi C, O'Hagan RC, Toniatti C. Genther Williams SM, et al. Cancer Cell Int. 2015 Feb 4;15(1):14. doi: 10.1186/s12935-015-0162-8. eCollection 2015. Cancer Cell Int. 2015. PMID: 25685067 Free PMC article. - Replication-induced DNA damage after PARP inhibition causes G2 delay, and cell line-dependent apoptosis, necrosis and multinucleation.
Dale Rein I, Solberg Landsverk K, Micci F, Patzke S, Stokke T. Dale Rein I, et al. Cell Cycle. 2015;14(20):3248-60. doi: 10.1080/15384101.2015.1085137. Cell Cycle. 2015. PMID: 26312527 Free PMC article. - DNA repair in cancer: emerging targets for personalized therapy.
Abbotts R, Thompson N, Madhusudan S. Abbotts R, et al. Cancer Manag Res. 2014 Feb 19;6:77-92. doi: 10.2147/CMAR.S50497. eCollection 2014. Cancer Manag Res. 2014. PMID: 24600246 Free PMC article. Review. - Development of synthetic lethality anticancer therapeutics.
Fang B. Fang B. J Med Chem. 2014 Oct 9;57(19):7859-73. doi: 10.1021/jm500415t. Epub 2014 Jun 13. J Med Chem. 2014. PMID: 24893124 Free PMC article. Review. - Preclinical evaluation of the PARP inhibitor BMN-673 for the treatment of ovarian clear cell cancer.
Wilkerson PM, Dedes KJ, Samartzis EP, Dedes I, Lambros MB, Natrajan R, Gauthier A, Piscuoglio S, Töpfer C, Vukovic V, Daley F, Weigelt B, Reis-Filho JS. Wilkerson PM, et al. Oncotarget. 2017 Jan 24;8(4):6057-6066. doi: 10.18632/oncotarget.14011. Oncotarget. 2017. PMID: 28002809 Free PMC article.
References
- Aaltonen LA, Salovaara R, Kristo P, Canzian F, Hemminki A, Peltomaki P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338(21):1481–7. - PubMed
- Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352(18):1851–60. - PubMed
- Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342(2):69–77. - PubMed
- Greenson JK, Bonner JD, Ben-Yzhak O, Cohen HI, Miselevich I, Resnick MB, et al. Phenotype of microsatellite unstable colorectal carcinomas: Well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am J Surg Pathol. 2003;27(5):563–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA046592/CA/NCI NIH HHS/United States
- T32 HG000040/HG/NHGRI NIH HHS/United States
- UL1RR024986/RR/NCRR NIH HHS/United States
- R03 CA130045/CA/NCI NIH HHS/United States
- R01 CA081488/CA/NCI NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- 1R01CA81488/CA/NCI NIH HHS/United States
- 5P30CA46592/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous