Deficiency of sorting nexin 27 (SNX27) leads to growth retardation and elevated levels of N-methyl-D-aspartate receptor 2C (NR2C) - PubMed (original) (raw)
Deficiency of sorting nexin 27 (SNX27) leads to growth retardation and elevated levels of N-methyl-D-aspartate receptor 2C (NR2C)
Lei Cai et al. Mol Cell Biol. 2011 Apr.
Abstract
Phox (PX) domain-containing sorting nexins (SNXs) are emerging as important regulators of endocytic trafficking. Sorting nexin 27 (SNX27) is unique, as it contains a PDZ (Psd-95/Dlg/ZO1) domain. We show here that SNX27 is primarily targeted to the early endosome by interaction of its PX domain with PtdIns(3)P. Although targeted ablation of the SNX27 gene in mice did not significantly affect growth and survival during embryonic development, SNX27 plays an essential role in postnatal growth and survival. N-Methyl-d-aspartate (NMDA) receptor 2C (NR2C) was identified as a novel SNX27-interacting protein, and this interaction is mediated by the PDZ domain of SNX27 and the C-terminal PDZ-binding motif of NR2C. Increased NR2C expression levels, together with impaired NR2C endocytosis in SNX27(-/-) neurons, indicate that SNX27 may function to regulate endocytosis and/or endosomal sorting of NR2C. This is consistent with a role of SNX27 as a general regulator for sorting of membrane proteins containing a PDZ-binding motif, and its absence may alter the trafficking of these proteins, leading to growth and survival defects.
Figures
Fig. 1.
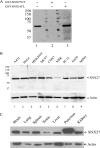
SNX27 antibodies revealed that SNX27 is widely expressed. (A) Affinity-purified rabbit antibodies against SNX27 were used to probe the membrane blot containing 50 μg A431 cell lysate resolved by SDS-PAGE. The antibodies were used without blocking protein (lane 1) or preincubated with GST-SNX27NT (lane 2) or with GST-SNX16FL (lane 3). (B) Forty micrograms of lysate derived from the indicated cultured cells of human (lanes 1 to 4), monkey (lane 5), rat (lane 6), and mouse (lanes 7 to 9) origin was loaded into each lane and resolved by SDS-PAGE, followed by immunoblotting analysis using anti-SNX27 antibodies. SNX27 (60 kDa) was detected in all the cell lines. The immunoblot was also probed with an antibody against β-actin as a loading control. (C) Forty micrograms of lysates derived from the indicated rat tissues was resolved by SDS-PAGE, followed by immunoblotting analysis using SNX27 antibodies. The immunoblot was also probed with an antibody against β-actin as a loading control.
Fig. 2.
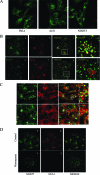
Endogenous SNX27 is enriched in the early endosome in a PtdIns(3)P-dependent manner. (A) HeLa, A431, and NIH 3T3 cells were fixed and processed for indirect immunofluorescence assay using a rabbit polyclonal antibody against SNX27 (revealed by fluorescein isothiocyanate [FITC]-conjugated goat polyclonal antibody against rabbit IgG). Bar, 10 μm. (B) A431 cells were fixed and processed for indirect immunofluorescence using a rabbit polyclonal antibody against SNX27 (revealed by FITC-conjugated goat polyclonal antibody against rabbit IgG) (a and e), a mouse monoclonal antibody against EEA1 (b), Golgi protein GS28 (f), or LAMP2 (g) (revealed by Alexa Fluor 555-conjugated goat polyclonal antibody against mouse IgG). The presence of yellow labeling indicates overlap in the merged images (d and h). Magnified images of the boxed regions in panels c and g are shown in panels d and h, respectively. (C) A431 cells were fixed and processed for indirect immunofluorescence assay using rabbit polyclonal antibody against SNX27 and mouse monoclonal antibody against α-adaptin and β-adaptin. SNX27 demonstrated colocalization with α-adaptin and β-adaptin of AP2. Magnified images of the boxed regions are shown in the right panels. (D) A431 cells were cultured in the absence of serum for 3 h, followed by treatment with (c and d) or without (a and b) wortmannin (25 nM) for 15 min. The cells were then fixed and processed for indirect immunofluorescence microscopy to detect SNX27 (a and c) and EEA1 (b and d). Like EEA1, endosomal association of SNX27 was abrogated upon treatment with wortmannin. Bars, 10 μm.
Fig. 3.
Generation of SNX27-deficient mice. (A) Schematic drawing of the SNX27 genomic fragment and the targeting vector. (B) Genotyping of SNX27 knockouts. Shown is PCR analysis of genomic DNA isolated from mouse tails for detection of SNX27+/+, SNX27+/−, and SNX27−/− mice. (C) Immunoblotting analysis of tissue lysates derived from the indicated mice using SNX27 antibodies, showing complete absence of SNX27 in the SNX27 knockout mice.
Fig. 4.
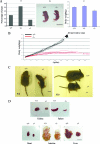
SNX27 deficiency caused growth retardation and reduced survival during embryonic development and severe postnatal growth retardation and lethality of newborn pups. (A) (a) Among 152 newborn pups, the ratio of SNX27−/− mice is only 16%, lower than the expected 25%, indicating the loss of some SNX27−/− embryos during development. Among 152 newborn pups, 25 are SNX27−/−, 44 are SNX27+/+, and 83 are SNX27+/−. (b) Newborn SNX27−/− mice are smaller than the wild-type pups in the same litter. (c) Body weight comparison among newborn SNX27+/+, SNX27+/−, and SNX27−/− mice, showing that SNX27−/− mice are significantly lighter. n = 10. Shown are means ± standard deviations. (B) The body weights during postnatal growth of SNX27−/− and SNX27+/+ pups were plotted as a function of time, showing severely delayed growth in body weight of SNX27−/− pups. All SNX27−/− mice died before being weaned. (C) (Left) Images of SNX27−/− and wild-type mice from the same litter at 10 days after birth (P10). (Right) Images of SNX27−/− and wild-type mice from the same litter at 20 days after birth, showing the dramatically reduced size of the former. (D) Images of major organs from SNX27−/− and wild-type micee at 10 days after birth, showing reduced sizes of multiple organs of the SNX27−/− mouse.
Fig. 5.
The PDZ domain of SNX27 is critical for interaction with NR2C. (A) Six SNX27 constructs (schematically depicted) were cloned in the pGBKT7 vector fused to GAL4BD and transformed into yeast AH109. The purified NR2C-CT in pGADT7 was retransformed into yeast Y187. (B) The indicated AH109 and Y187 yeast cells were mated and plated on leucine- and tryptophan-free SD plates (SD/−Leu/−Trp). To confirm interaction, mated yeast cells were grown on the highest-stringency medium lacking leucine, tryptophan, histidine, and adenine (SD/−Leu/−Trp/−His/−Ade).
Fig. 6.
The PDZ domain of SNX27 mediates interaction with the C-terminal PDZ-binding motif of NR2C. (A) Alignment of the C-terminal amino acid sequences of human, rat, and mouse NR2C showing the conserved class I PDZ-binding motif (SEV). (B) Diagrammatic representation of the NR2C and SNX27a deletion mutants. (C) HEK-293 cells were cotransfected with HA-SNX27 (or its indicated mutants) and Myc-NR2C-CT or Myc-NR2C-CTΔSEV. Total cell lysates were prepared and incubated with monoclonal antibody against Myc cross-linked to protein G-Sepharose (BD). The immunoprecipitates were resolved by SDS-PAGE and then processed for immunoblotting using rabbit polyclonal antibody against the HA tag (top) to assess the efficiency of coimmunoprecipitation as a measure of interaction. Following stripping, the immunoblots were reprobed with rabbit polyclonal antibody against the Myc tag (middle) to ensure the efficiency of immunoprecipitation of the Myc-tagged proteins. Five percent of the lysates used in the immunoprecipitations were also analyzed for the expression of HA-tagged SNX27 or its mutants (bottom). IP, immunoprecipitation; WB, Western blotting.
Fig. 7.
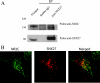
Interaction of endogenous SNX27 and NR2C; SNX27 deficiency leads to enhanced levels of NR2C. (A) Mouse brain extracts were immunoprecipitated with antibodies against SNX27, and the immunoprecipitates were resolved by SDS-PAGE, followed by immunoblot analysis using antibodies against SNX27 and NR2C. A noticeable amount of NR2C was coprecipitated with SNX27. (B) SNX27 colocalized with NR2C in cultured neurons. Primary cerebellar neurons from wild-type mice were cultured for 7 days and stained with SNX27 and NR2C. Colocalization of NR2C and SNX27 was observed in the merged image.
Fig. 8.
Elevated NR2C expression and impaired NR2C endocytosis in SNX27-deficient cerebellar neurons. (A) Brain lysates from newborn wild-type or SNX27−/− pups were resolved by SDS-PAGE, followed by immunoblotting using antibodies against SNX27, NR1, NR2C, or β-actin. The level of NR2C was increased in SNX27-negative brain lysate. The quantitative results of NR2C levels normalized to β-actin in four independent experiments are presented as means and standard deviations; the levels in the wild type were arbitrarily set as 100%. (B) NR2C mRNA transcript levels in wild-type or SNX27−/− brains were quantified by real-time PCR. The mRNA transcript levels in wild-type and SNX27−/− brains were not significantly different. The experiment was repeated three times, and similar results were obtained. (C) (Left) Cryosections of cerebellum from SNX27-deficient and wild-type mice stained with NR2C antibody. Significantly more NR2C was expressed in SNX27-deficient cerebellum than in wild-type cerebellum. (Middle) Quantitation of immunofluorescence intensity shown in the cryosections. (Right) Primary cerebellar neurons from SNX27-deficient and wild-type mice were cultured for 7 days and stained with NR2C antibody. Similar to panel A, significantly more NR2C was expressed in SNX27-deficient cerebellar neurons than in wild-type cerebellar neurons. (D) Primary cortical neurons from SNX27+/+ and SNX27−/− mice were transfected with Myc-NR2C on day 4 in vitro (4 DIV) and pulse-chased with anti-Myc antibody on 6 DIV. Surface NR2C receptors were labeled with AF-488 secondary antibody without permeabilization, while internalized NR2C receptors were labeled with AF-555 secondary antibody following permeabilization. Reduced NR2C endocytosis was observed in SNX27-deficient neurons compared to wild-type neurons.
Similar articles
- A role for novel lipid interactions in the dynamic recruitment of SNX27 to the T-cell immune synapse.
Tello-Lafoz M, Ghai R, Collins B, Mérida I. Tello-Lafoz M, et al. Bioarchitecture. 2014;4(6):215-20. doi: 10.1080/19490992.2015.1031950. Epub 2015 May 21. Bioarchitecture. 2014. PMID: 25996807 Free PMC article. - Sorting nexin 27 (SNX27) associates with zonula occludens-2 (ZO-2) and modulates the epithelial tight junction.
Zimmerman SP, Hueschen CL, Malide D, Milgram SL, Playford MP. Zimmerman SP, et al. Biochem J. 2013 Oct 1;455(1):95-106. doi: 10.1042/BJ20121755. Biochem J. 2013. PMID: 23826934 Free PMC article. - A molecular code for endosomal recycling of phosphorylated cargos by the SNX27-retromer complex.
Clairfeuille T, Mas C, Chan AS, Yang Z, Tello-Lafoz M, Chandra M, Widagdo J, Kerr MC, Paul B, Mérida I, Teasdale RD, Pavlos NJ, Anggono V, Collins BM. Clairfeuille T, et al. Nat Struct Mol Biol. 2016 Oct;23(10):921-932. doi: 10.1038/nsmb.3290. Epub 2016 Sep 5. Nat Struct Mol Biol. 2016. PMID: 27595347 - Endosomal Sorting Protein SNX27 and Its Emerging Roles in Human Cancers.
Deb S, Sun J. Deb S, et al. Cancers (Basel). 2022 Dec 22;15(1):70. doi: 10.3390/cancers15010070. Cancers (Basel). 2022. PMID: 36612066 Free PMC article. Review. - Emerging Role of Sorting Nexin 17 in Human Health and Disease.
Chen J, Su YH, Wang M, Zhang YC. Chen J, et al. Curr Protein Pept Sci. 2024;25(10):814-825. doi: 10.2174/0113892037284582240522155112. Curr Protein Pept Sci. 2024. PMID: 38874037 Review.
Cited by
- PX-FERM proteins: A link between endosomal trafficking and signaling?
Ghai R, Collins BM. Ghai R, et al. Small GTPases. 2011 Sep;2(5):259-263. doi: 10.4161/sgtp.2.5.17276. Epub 2011 Sep 1. Small GTPases. 2011. PMID: 22292128 Free PMC article. - Snx27 Deletion Promotes Recovery From Spinal Cord Injury by Neuroprotection and Reduces Macrophage/Microglia Proliferation.
Zeng Y, Wang N, Guo T, Zheng Q, Wang S, Wu S, Li X, Wu J, Chen Z, Xu H, Wang X, Lin B. Zeng Y, et al. Front Neurol. 2018 Dec 13;9:1059. doi: 10.3389/fneur.2018.01059. eCollection 2018. Front Neurol. 2018. PMID: 30619032 Free PMC article. - Sorting nexin 27 protein regulates trafficking of a p21-activated kinase (PAK) interacting exchange factor (β-Pix)-G protein-coupled receptor kinase interacting protein (GIT) complex via a PDZ domain interaction.
Valdes JL, Tang J, McDermott MI, Kuo JC, Zimmerman SP, Wincovitch SM, Waterman CM, Milgram SL, Playford MP. Valdes JL, et al. J Biol Chem. 2011 Nov 11;286(45):39403-16. doi: 10.1074/jbc.M111.260802. Epub 2011 Sep 18. J Biol Chem. 2011. PMID: 21926430 Free PMC article. - Understanding the genetic mechanisms and cognitive impairments in Down syndrome: towards a holistic approach.
Abukhaled Y, Hatab K, Awadhalla M, Hamdan H. Abukhaled Y, et al. J Neurol. 2024 Jan;271(1):87-104. doi: 10.1007/s00415-023-11890-0. Epub 2023 Aug 10. J Neurol. 2024. PMID: 37561187 Free PMC article. Review. - Partial loss of Sorting Nexin 27 resembles age- and Down syndrome-associated T cell dysfunctions.
Rodriguez-Rodriguez C, González-Mancha N, Ochoa-Echeverría A, Liébana R, Merida I. Rodriguez-Rodriguez C, et al. Immun Ageing. 2024 Jan 2;21(1):2. doi: 10.1186/s12979-023-00402-3. Immun Ageing. 2024. PMID: 38166948 Free PMC article.
References
- Aridor M., Hannan L. A. 2000. Traffic jam: a compendium of human diseases that affect intracellular transport processes. Traffic 1:836–851 - PubMed
- Corvera S., D'Arrigo A., Stenmark H. 1999. Phosphoinositides in membrane traffic. Curr. Opin. Cell Biol. 11:460–465 - PubMed
- Cullen P. J. 2008. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat. Rev. Mol. Cell Biol. 9:574–582 - PubMed
- Dell'Angelica E. C., Shotelersuk V., Aguilar R. C., Gahl W. A., Bonifacino J. S. 1999. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol. Cell 3:11–21 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases