Circulating tumor cells: approaches to isolation and characterization - PubMed (original) (raw)
Review
Circulating tumor cells: approaches to isolation and characterization
Min Yu et al. J Cell Biol. 2011.
Abstract
Circulating tumor cells (CTCs) shed from primary and metastatic cancers are admixed with blood components and are thus rare, making their isolation and characterization a major technological challenge. CTCs hold the key to understanding the biology of metastasis and provide a biomarker to noninvasively measure the evolution of tumor genotypes during treatment and disease progression. Improvements in technologies to yield purer CTC populations amenable to better cellular and molecular characterization will enable a broad range of clinical applications, including early detection of disease and the discovery of biomarkers to predict treatment responses and disease progression.
Figures
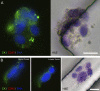
Figure 1.
Micrographs of CTCs captured from patients’ blood using an anti-EpCAM–coated CTC-chip. (A–C) Combined fluorescent and reflected light micrographs of a cytokeratin 7/8 (green)–stained CTC captured from breast cancer patient blood and a contaminating CD45-positive (red) white blood cell (A) and cytokeratin 7/8 (green, B)– and PSA (green, C)-stained CTCs captured from a prostate cancer patient. (D and E) HER2 (green, D)- and cytokeratin 7/8 (green, E)–stained CTCs captured from a breast cancer patient. (F and G) Individual and merged fluorescent micrographs of CTCs captured from prostate cancer patients’ blood stained positive for PSA (green) and Ki-67 (red, proliferative marker, F), and PSA (green) and M30 (red, apoptotic marker) demonstrated the heterogeneity in CTCs (G). In all panels, the nuclei are stained with DAPI (blue). CK7/8, cytokeratin 7/8. Bars, 10 µm.
Figure 2.
Illustration of current and potential applications of CTC technologies. The peripheral blood of a cancer patient is collected and processed through various CTC isolation technologies. CTCs are captured along with contaminating leukocytes. Immunostaining for specific markers and FISH for genomic amplification and translocation can be applied to CTCs. DNA or RNA can be extracted from the CTCs and subjected to sequencing, quantitative RT-PCR (qRT-PCR), and potential expression profile analysis. Viable cells can be released and propagated in cell culture.
Figure 3.
CTC clusters captured from lung cancer patients’ blood using an anti-EpCAM–coated HB-chip. (A and B) Fluorescent micrographs of cytokeratin 7/8 (CK; green)–, CD45 (red)-, and DAPI (blue)-stained CTC clusters taken at different focal planes and corresponding hematoxylin and eosin (H&E) stains are shown. Bars, 10 µm.
Similar articles
- Microfluidics and circulating tumor cells.
Dong Y, Skelley AM, Merdek KD, Sprott KM, Jiang C, Pierceall WE, Lin J, Stocum M, Carney WP, Smirnov DA. Dong Y, et al. J Mol Diagn. 2013 Mar;15(2):149-57. doi: 10.1016/j.jmoldx.2012.09.004. Epub 2012 Dec 22. J Mol Diagn. 2013. PMID: 23266318 Review. - Isolation of rare circulating tumour cells in cancer patients by microchip technology.
Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, Ryan P, Balis UJ, Tompkins RG, Haber DA, Toner M. Nagrath S, et al. Nature. 2007 Dec 20;450(7173):1235-9. doi: 10.1038/nature06385. Nature. 2007. PMID: 18097410 Free PMC article. - Circulating tumor cells: a window into cancer biology and metastasis.
Maheswaran S, Haber DA. Maheswaran S, et al. Curr Opin Genet Dev. 2010 Feb;20(1):96-9. doi: 10.1016/j.gde.2009.12.002. Epub 2010 Jan 12. Curr Opin Genet Dev. 2010. PMID: 20071161 Free PMC article. Review. - Affinity Versus Label-Free Isolation of Circulating Tumor Cells: Who Wins?
Murlidhar V, Rivera-Báez L, Nagrath S. Murlidhar V, et al. Small. 2016 Sep;12(33):4450-63. doi: 10.1002/smll.201601394. Epub 2016 Jul 20. Small. 2016. PMID: 27436104 Review. - Improved detection by ensemble-decision aliquot ranking of circulating tumor cells with low numbers of a targeted surface antigen.
Johnson ES, Anand RK, Chiu DT. Johnson ES, et al. Anal Chem. 2015 Sep 15;87(18):9389-95. doi: 10.1021/acs.analchem.5b02241. Epub 2015 Aug 31. Anal Chem. 2015. PMID: 26302174
Cited by
- Tunable nanostructured coating for the capture and selective release of viable circulating tumor cells.
Reátegui E, Aceto N, Lim EJ, Sullivan JP, Jensen AE, Zeinali M, Martel JM, Aranyosi AJ, Li W, Castleberry S, Bardia A, Sequist LV, Haber DA, Maheswaran S, Hammond PT, Toner M, Stott SL. Reátegui E, et al. Adv Mater. 2015 Mar 4;27(9):1593-9. doi: 10.1002/adma.201404677. Epub 2015 Jan 15. Adv Mater. 2015. PMID: 25640006 Free PMC article. - CD133+CD54+CD44+ circulating tumor cells as a biomarker of treatment selection and liver metastasis in patients with colorectal cancer.
Fang C, Fan C, Wang C, Huang Q, Meng W, Yu Y, Yang L, Peng Z, Hu J, Li Y, Mo X, Zhou Z. Fang C, et al. Oncotarget. 2016 Nov 22;7(47):77389-77403. doi: 10.18632/oncotarget.12675. Oncotarget. 2016. PMID: 27764803 Free PMC article. - Protein disulfide isomerases in the endoplasmic reticulum promote anchorage-independent growth of breast cancer cells.
Wise R, Duhachek-Muggy S, Qi Y, Zolkiewski M, Zolkiewska A. Wise R, et al. Breast Cancer Res Treat. 2016 Jun;157(2):241-252. doi: 10.1007/s10549-016-3820-1. Epub 2016 May 9. Breast Cancer Res Treat. 2016. PMID: 27161215 Free PMC article. - RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis.
Yu M, Ting DT, Stott SL, Wittner BS, Ozsolak F, Paul S, Ciciliano JC, Smas ME, Winokur D, Gilman AJ, Ulman MJ, Xega K, Contino G, Alagesan B, Brannigan BW, Milos PM, Ryan DP, Sequist LV, Bardeesy N, Ramaswamy S, Toner M, Maheswaran S, Haber DA. Yu M, et al. Nature. 2012 Jul 26;487(7408):510-3. doi: 10.1038/nature11217. Nature. 2012. PMID: 22763454 Free PMC article. - High-Grade Serous Ovarian Cancer-A Risk Factor Puzzle and Screening Fugitive.
Wilczyński J, Paradowska E, Wilczyński M. Wilczyński J, et al. Biomedicines. 2024 Jan 19;12(1):229. doi: 10.3390/biomedicines12010229. Biomedicines. 2024. PMID: 38275400 Free PMC article. Review.
References
- Alix-Panabières C., Vendrell J.P., Slijper M., Pellé O., Barbotte E., Mercier G., Jacot W., Fabbro M., Pantel K. 2009. Full-length cytokeratin-19 is released by human tumor cells: a potential role in metastatic progression of breast cancer. Breast Cancer Res. 11:R39 10.1186/bcr2326 - DOI - PMC - PubMed
- Allan A.L., Vantyghem S.A., Tuck A.B., Chambers A.F., Chin-Yee I.H., Keeney M. 2005. Detection and quantification of circulating tumor cells in mouse models of human breast cancer using immunomagnetic enrichment and multiparameter flow cytometry. Cytometry A. 65:4–14 - PubMed
- Allard W.J., Matera J., Miller M.C., Repollet M., Connelly M.C., Rao C., Tibbe A.G., Uhr J.W., Terstappen L.W. 2004. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 10:6897–6904 10.1158/1078-0432.CCR-04-0378 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources