Promotion of experimental thrombus formation by the procoagulant activity of breast cancer cells - PubMed (original) (raw)
Promotion of experimental thrombus formation by the procoagulant activity of breast cancer cells
M A Berny-Lang et al. Phys Biol. 2011 Feb.
Abstract
The routine observation of tumor emboli in the peripheral blood of patients with carcinomas raises questions about the clinical relevance of these circulating tumor cells. Thrombosis is a common clinical manifestation of cancer, and circulating tumor cells may play a pathogenetic role in this process. The presence of coagulation-associated molecules on cancer cells has been described, but the mechanisms by which circulating tumor cells augment or alter coagulation remains unclear. In this study we utilized suspensions of a metastatic adenocarcinoma cell line, MDA-MB-231, and a non-metastatic breast epithelial cell line, MCF-10A, as models of circulating tumor cells to determine the thrombogenic activity of these blood-foreign cells. In human plasma, both metastatic MDA-MB-231 cells and non-metastatic MCF-10A cells significantly enhanced clotting kinetics. The effect of MDA-MB-231 and MCF-10A cells on clotting times was cell number-dependent and inhibited by a neutralizing antibody to tissue factor (TF) as well as inhibitors of activated factor X and thrombin. Using fluorescence microscopy, we found that both MDA-MB-231 and MCF-10A cells supported the binding of fluorescently labeled thrombin. Furthermore, in a model of thrombus formation under pressure-driven flow, MDA-MB-231 and MCF-10A cells significantly decreased the time to occlusion. Our findings indicate that the presence of breast epithelial cells in blood can stimulate coagulation in a TF-dependent manner, suggesting that tumor cells that enter the circulation may promote the formation of occlusive thrombi under shear flow conditions.
Figures
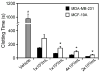
Figure 1. Characterization of the procoagulant activity of breast epithelial cells
Human sodium citrate-anticoagulated plasma was incubated with vehicle or suspensions of cultured MDA-MB-231 or MCF-10A cells (1×103 – 2×105/mL) for 3 minutes at 37°C. Coagulation of plasma was initiated by recalcification using 16.7 mM CaCl2 (final concentration) and clotting times were recorded on a coagulometer. Data are reported as mean ± SEM, from 6-8 experiments. In comparison to vehicle, clotting times were significantly shortened at all MDA-MB-231 or MCF-10A cell numbers, #P<0.05. *P<0.05 versus corresponding MDA-MB-231 cell concentration.
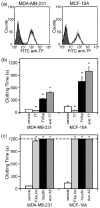
Figure 2. Characterization of the procoagulant activity of breast epithelial cells
(a) Cultured MDA-MB-231 or MCF-10A cells (1×106/mL) were labeled with a FITC-conjugated anti-TF antibody (1 μg/mL) and analyzed by flow cytometry. Shaded curves represent background fluorescence of unlabeled cells; white curves represent shift in fluorescence in the presence the anti-TF antibody. Representative curves from two or more independent experiments are shown. (b&c) Human sodium citrate-anticoagulated plasma was pretreated with (b) vehicle; TF (TF,10 pM); the TF pathway inhibitor, FVIIai (20 μg/mL); or a neutralizing antibody to TF (anti-TF, 20 μg/mL) or (c) vehicle; the FXa inhibitor, rivaroxaban (FXa inh, 10 μM); the thrombin inhibitor, hirudin (20 μg/mL); or the phosphatidylserine binding protein, annexin A5 (Ann A5, 10 μg/mL). Cultured MDA-MB-231 or MCF-10A cells were added to treated plasma at 1×104/mL. After 3 minutes of incubation at 37°C, coagulation was initiated by addition of 16.7 mM CaCl2 and clotting times were recorded. Data are reported as mean ± SEM, from 4-8 experiments. If clotting did not occur during 20 minutes of observation, experiments were terminated and a clotting time of 20 minutes was recorded. *P<0.05 versus vehicle treatment.
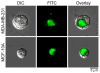
Figure 3. Cultured breast epithelial cells bind thrombin under procoagulant conditions
Human sodium citrate-anticoagulated plasma was incubated with suspended MDA-MB-231 or MCF-10A cells (2×105/mL) for 3 minutes at 37°C in the presence of OG-488 active-site labeled thrombin (1 μM). Plasma was pretreated with GPRP (10 mM), an inhibitor of fibrin polymerization, to prevent complete gelation. Coagulation was initiated by addition of 16.7 mM CaCl2 and plasma was sampled 5 minutes later. Samples were imaged by DIC and fluorescence microscopy, a representative image of a MDA-MB-231 and MCF-10A cell binding thrombin is shown. OG-488 thrombin fluorescence is indicated in green.
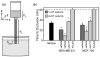
Figure 4. Cultured breast epithelial cells promote TF-dependent occlusive thrombus formation in flowing blood, ex vivo
Human sodium citrate-anticoagulated whole blood was mixed with vehicle, MDA-MB-231 or MCF-10A cells (4×104 or 1×103/mL) for 5 minutes at room temperature. In selected experiments, blood was treated with a neutralizing antibody to TF (anti-TF, 20 μg/mL) or the thrombin inhibitor, hirudin (20 μg/mL), in the presence of MDA-MB-231 or MCF-10A cells. (a) Treated blood was recalcified with CaCl2 and MgCl2 (final concentration 7.5 and 3.75 mM, respectively), added to a reservoir to a set height (hb), and allowed to drain through collagen-coated capillaries into a PBS bath as shown. (b) The time to thrombotic occlusion (time until blood ceased to flow from the capillary) was recorded. The height of blood in the reservoir was maintained at 1.5 cm, yielding an initial shear rate of 285 s-1 in the 0.2 × 2.0 × 50 mm collagen-coated capillary, as described in Materials and Methods. Data are mean ± SEM from 3 or more experiments. *P<0.05 versus vehicle treatment in the absence of cells. #P<0.05 versus vehicle treatment of corresponding cell type at 4×104/mL.
Similar articles
- Breast-cancer extracellular vesicles induce platelet activation and aggregation by tissue factor-independent and -dependent mechanisms.
Gomes FG, Sandim V, Almeida VH, Rondon AMR, Succar BB, Hottz ED, Leal AC, Verçoza BRF, Rodrigues JCF, Bozza PT, Zingali RB, Monteiro RQ. Gomes FG, et al. Thromb Res. 2017 Nov;159:24-32. doi: 10.1016/j.thromres.2017.09.019. Epub 2017 Sep 21. Thromb Res. 2017. PMID: 28950217 - Enhanced cell-associated plasminogen activator pathway but not coagulation pathway activity contributes to motility in metastatic breast cancer cells.
Carter JC, Campbell RA, Gibbons JA, Gramling MW, Wolberg AS, Church FC. Carter JC, et al. J Thromb Haemost. 2010 Jun;8(6):1323-32. doi: 10.1111/j.1538-7836.2010.03825.x. Epub 2010 Feb 24. J Thromb Haemost. 2010. PMID: 20180817 - Opposite regulation by PI3K/Akt and MAPK/ERK pathways of tissue factor expression, cell-associated procoagulant activity and invasiveness in MDA-MB-231 cells.
Hu C, Huang L, Gest C, Xi X, Janin A, Soria C, Li H, Lu H. Hu C, et al. J Hematol Oncol. 2012 Jul 11;5:16. doi: 10.1186/1756-8722-5-16. J Hematol Oncol. 2012. PMID: 22534171 Free PMC article. - The Role of Fluid Shear and Metastatic Potential in Breast Cancer Cell Migration.
Riehl BD, Kim E, Lee JS, Duan B, Yang R, Donahue HJ, Lim JY. Riehl BD, et al. J Biomech Eng. 2020 Oct 1;142(10):101001. doi: 10.1115/1.4047076. J Biomech Eng. 2020. PMID: 32346724 Free PMC article.
Cited by
- Do circulating tumor cells play a role in coagulation and thrombosis?
Tormoen GW, Haley KM, Levine RL, McCarty OJ. Tormoen GW, et al. Front Oncol. 2012 Sep 10;2:115. doi: 10.3389/fonc.2012.00115. eCollection 2012. Front Oncol. 2012. PMID: 22973557 Free PMC article. - The role of coagulation and platelets in colon cancer-associated thrombosis.
Mitrugno A, Tassi Yunga S, Sylman JL, Zilberman-Rudenko J, Shirai T, Hebert JF, Kayton R, Zhang Y, Nan X, Shatzel JJ, Esener S, Duvernay MT, Hamm HE, Gruber A, Williams CD, Takata Y, Armstrong R, Morgan TK, McCarty OJT. Mitrugno A, et al. Am J Physiol Cell Physiol. 2019 Feb 1;316(2):C264-C273. doi: 10.1152/ajpcell.00367.2018. Epub 2018 Nov 21. Am J Physiol Cell Physiol. 2019. PMID: 30462538 Free PMC article. - A Direct Podocalyxin-Dynamin-2 Interaction Regulates Cytoskeletal Dynamics to Promote Migration and Metastasis in Pancreatic Cancer Cells.
Wong BS, Shea DJ, Mistriotis P, Tuntithavornwat S, Law RA, Bieber JM, Zheng L, Konstantopoulos K. Wong BS, et al. Cancer Res. 2019 Jun 1;79(11):2878-2891. doi: 10.1158/0008-5472.CAN-18-3369. Epub 2019 Apr 11. Cancer Res. 2019. PMID: 30975647 Free PMC article. - Tamoxifen induces hypercoagulation and alterations in ERα and ERβ dependent on breast cancer sub-phenotype ex vivo.
Pather K, Augustine TN. Pather K, et al. Sci Rep. 2020 Nov 6;10(1):19256. doi: 10.1038/s41598-020-75779-y. Sci Rep. 2020. PMID: 33159119 Free PMC article. - Development of coagulation factor probes for the identification of procoagulant circulating tumor cells.
Tormoen GW, Cianchetti FA, Bock PE, McCarty OJ. Tormoen GW, et al. Front Oncol. 2012 Sep 6;2:110. doi: 10.3389/fonc.2012.00110. eCollection 2012. Front Oncol. 2012. PMID: 22973554 Free PMC article.
References
- Braun S, Pantel K, Muller P, Janni W, Hepp F, Kentenich CR, Gastroph S, Wischnik A, Dimpfl T, Kindermann G, et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med. 2000;342:525–33. - PubMed
- Bouillard JB, Bouillard S. De l’Obliteration des veines et de son influence sur la formation des hydropisies partielles: consideration sur la hydropisies passive et general. Arch Gen Med. 1823;1:188–204.
- Trousseau A. Clinique Medicale de l’Hotel-Dieu de Paris. Paris, France: The Syndenham Society; 1865. Phlegmasia alba dolens; pp. 654–712.
- Heit JA, Mohr DN, Silverstein MD, Petterson TM, O’Fallon WM, Melton LJ., 3rd Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med. 2000;160:761–8. - PubMed
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–22. - PubMed
Publication types
MeSH terms
Grants and funding
- R01HL038779/HL/NHLBI NIH HHS/United States
- R37 HL071544/HL/NHLBI NIH HHS/United States
- R01HL101972/HL/NHLBI NIH HHS/United States
- R01 HL101972/HL/NHLBI NIH HHS/United States
- T32 HL00778118/HL/NHLBI NIH HHS/United States
- R37HL071544/HL/NHLBI NIH HHS/United States
- 1U54CA143906-01/CA/NCI NIH HHS/United States
- R01 HL038779/HL/NHLBI NIH HHS/United States
- U54 CA143906/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous