Hypertrophic cardiomyopathy in young Maine Coon cats caused by the p.A31P cMyBP-C mutation--the clinical significance of having the mutation - PubMed (original) (raw)
Hypertrophic cardiomyopathy in young Maine Coon cats caused by the p.A31P cMyBP-C mutation--the clinical significance of having the mutation
Mia T N Godiksen et al. Acta Vet Scand. 2011.
Abstract
Background: In Maine Coon (MC) cats the c.91G > C mutation in the gene MYBPC3, coding for cardiac myosin binding protein C (cMyBP-C), is associated with feline hypertrophic cardiomyopathy (fHCM). The mutation causes a substitution of an alanine for a proline at residue 31 (p.A31P) of cMyBP-C. The pattern of inheritance has been considered autosomal dominant based on a single pedigree. However, larger studies are needed to establish the significance of cats being heterozygous or homozygous for the mutation with respect to echocardiographic indices and the probability of developing fHCM. The objective of the present study was to establish the clinical significance of being homozygous or heterozygous for the p.A31P cMyBP-C mutation in young to middle-aged cats.
Methods: The cohort consisted of 332 MC cats, 282 cats < 4 years (85%). All cats were examined by 2-D and M-mode echocardiography. DNA was extracted from blood samples or buccal swabs and screened for the p.A31P cMyBP-C mutation in exon 3 of the gene, using polymerase chain reaction followed by DNA sequencing.
Results: The fHCM prevalence was 6.3% in the cohort. Eighteen cats were homozygous and 89 cats were heterozygous for the mutation. The odds ratio for having fHCM for homozygous cats was 21.6 (95% confidence interval 7.01-66.2) - when the group of equivocal cats was categorized as non-affected. Overall, 50% of the cats that were homozygous for the mutation had fHCM. p.A31P heterozygosity was not associated with a significant odds ratio for fHCM. In cats in the 4 to 6 years of age range a similar, non significant, odds ratio was seen in heterozygous cats. Only two cats over four years were homozygous and both were diagnosed with fHCM.
Conclusion: As there is no significant odds ratio associated with being heterozygous for the pA31P cMyBP-C mutation at this age, the mutation must have a very low penetrance in this group. From our data it would appear that most MC cats that develop fHCM due to the p.A31P mutation prior to the age of approximately 6 years do so because they are homozygous for this mutation.
Figures
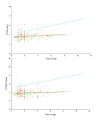
Figure 1
The correlation with age and diastolic IVS and LVFW measurements as a function of c.91C MYBPC3 alleles. Top: Plot of diastolic IVS correlation with age as a function numbers of c.91C MYBPC3 alleles. There was tendency to an increasing effect of p.A31P cMyBP-C homozygosity with age, though the tendency was not significant, ρ = 0.41, P = 0.09. There was only a low effect of being p.A31P cMyBP-C heterozygous, ρ = 0.09, P = 0.4. Bottom: Plot of diastolic LVFW correlation with age as a function of numbers of MYBPC3 c.91C alleles. The tendency was more weak than seen above, ρ = 0.2, P = 0.50. Red indicates the cMyBP-C wild type coordinates and the corresponding line of tendency. Green indicates p.A31P cMyBP-C heterozygous coordinates and the corresponding line of tendency. Blue indicates p.A31P cMyBP-C homozygous where and the corresponding line of tendency. Figure abbreviations: IVSd: diastolic interventricular septum, LVFWd: diastolic left ventricle free wall.
Figure 2
2-D echocardiographic recordings of papillary muscles from a normal and an fHCM affected cat. Left illustration: Right parasternal short axis of the left ventricle with papillary muscles view from a normal MC cat. Middle illustration: Right parasternal short axis of the left ventricle with papillary muscles view from a cat with severe hypertrophic cardiomyopathy. Right illustration: Gross heart specimen from a cat with severe concentric hypertrophy due to fHCM. Figure abbreviations: IVS: Intraventricular septum, LV: left ventricle and PW: posterior wall (free wall).
Figure 3
Illustration of M-mode echocardiographic recordings from a normal and an fHCM affected cat. Left illustration: M-mode echocardiogram recorded from an MC cat at the level of chordae tendinae shows normal left ventricular internal dimension, septum and posterior wall of the left ventricle. Right: M-mode echocardiogram from and an MC cat with severe fHCM with atrial fibrillation (HR 325/min), congestive heart failure and tromboembolic disease, recorded at the level of the chordae tendinae to assess left ventricular dimensions shows severe thickening of both the septum and posterior wall of the left ventricle. Figure abbreviations: IVS: Intraventricular septum, LV: left ventricle and PW: posterior wall.
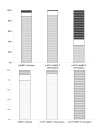
Figure 4
Distributions of fHCM and equivocal cases in respect to p.A31P cMyBP-C genotype status. A: Histogram of the distribution for the entire cohort. The group of p.A31P cMyBP-C homozygous cats had an increased proportion of fHCM cases. B. Histogram of the distribution for cats over 4 years of age. Except for the homozygous the distribution was very similar to the distribution shown in A. Parts of boxes marked with small dots represent fHCM negative cats. Blank parts of the boxes indicate equivocal cases. Box marked with black stripes indicate cases of fHCM.
Similar articles
- The genetic basis of hypertrophic cardiomyopathy in cats and humans.
Kittleson MD, Meurs KM, Harris SP. Kittleson MD, et al. J Vet Cardiol. 2015 Dec;17 Suppl 1(Suppl 1):S53-73. doi: 10.1016/j.jvc.2015.03.001. J Vet Cardiol. 2015. PMID: 26776594 Free PMC article. Review. - Genotype-phenotype correlation between the cardiac myosin binding protein C mutation A31P and hypertrophic cardiomyopathy in a cohort of Maine Coon cats: a longitudinal study.
Granström S, Godiksen MT, Christiansen M, Pipper CB, Martinussen T, Møgelvang R, Søgaard P, Willesen JL, Koch J. Granström S, et al. J Vet Cardiol. 2015 Dec;17 Suppl 1:S268-81. doi: 10.1016/j.jvc.2015.10.005. J Vet Cardiol. 2015. PMID: 26776585 - Prospective echocardiographic and tissue Doppler imaging screening of a population of Maine Coon cats tested for the A31P mutation in the myosin-binding protein C gene: a specific analysis of the heterozygous status.
Carlos Sampedrano C, Chetboul V, Mary J, Tissier R, Abitbol M, Serres F, Gouni V, Thomas A, Pouchelon JL. Carlos Sampedrano C, et al. J Vet Intern Med. 2009 Jan-Feb;23(1):91-9. doi: 10.1111/j.1939-1676.2008.0218.x. J Vet Intern Med. 2009. PMID: 19175727 - Association of A31P and A74T polymorphisms in the myosin binding protein C3 gene and hypertrophic cardiomyopathy in Maine Coon and other breed cats.
Wess G, Schinner C, Weber K, Küchenhoff H, Hartmann K. Wess G, et al. J Vet Intern Med. 2010 May-Jun;24(3):527-32. doi: 10.1111/j.1939-1676.2010.0514.x. Epub 2010 Apr 16. J Vet Intern Med. 2010. PMID: 20412438 - Genetics of feline hypertrophic cardiomyopathy.
Gil-Ortuño C, Sebastián-Marcos P, Sabater-Molina M, Nicolas-Rocamora E, Gimeno-Blanes JR, Fernández Del Palacio MJ. Gil-Ortuño C, et al. Clin Genet. 2020 Sep;98(3):203-214. doi: 10.1111/cge.13743. Epub 2020 Apr 1. Clin Genet. 2020. PMID: 32215921 Review.
Cited by
- Speckle tracking echocardiography in cats with preclinical hypertrophic cardiomyopathy.
Spalla I, Boswood A, Connolly DJ, Luis Fuentes V. Spalla I, et al. J Vet Intern Med. 2019 May;33(3):1232-1241. doi: 10.1111/jvim.15495. Epub 2019 Apr 16. J Vet Intern Med. 2019. PMID: 30993757 Free PMC article. - Precision medicine validation: identifying the _MYBPC_3 A31P variant with whole-genome sequencing in two Maine Coon cats with hypertrophic cardiomyopathy.
Ontiveros ES, Ueda Y, Harris SP, Stern JA; 99 Lives Consortium. Ontiveros ES, et al. J Feline Med Surg. 2019 Dec;21(12):1086-1093. doi: 10.1177/1098612X18816460. Epub 2018 Dec 18. J Feline Med Surg. 2019. PMID: 30558461 Free PMC article. - The genetic basis of hypertrophic cardiomyopathy in cats and humans.
Kittleson MD, Meurs KM, Harris SP. Kittleson MD, et al. J Vet Cardiol. 2015 Dec;17 Suppl 1(Suppl 1):S53-73. doi: 10.1016/j.jvc.2015.03.001. J Vet Cardiol. 2015. PMID: 26776594 Free PMC article. Review. - Influence of Signalment Variables on Body Weight-Normalized Echocardiographic Measurements of Heart Size in 56 169 Adult Unsedated Normal Pure-Bred Cats.
Häggström J, Ohlsson Å, Falk T, Wess G, Friederich J, Olsson U, Nilsfors L, Kresken JG, Högström K, Beijerink N, Rishniw M, Tidholm A, Ljungvall I. Häggström J, et al. J Vet Intern Med. 2025 Jul-Aug;39(4):e70146. doi: 10.1111/jvim.70146. J Vet Intern Med. 2025. PMID: 40447458 Free PMC article. - Genetic epidemiology of blood type, disease and trait variants, and genome-wide genetic diversity in over 11,000 domestic cats.
Anderson H, Davison S, Lytle KM, Honkanen L, Freyer J, Mathlin J, Kyöstilä K, Inman L, Louviere A, Chodroff Foran R, Forman OP, Lohi H, Donner J. Anderson H, et al. PLoS Genet. 2022 Jun 16;18(6):e1009804. doi: 10.1371/journal.pgen.1009804. eCollection 2022 Jun. PLoS Genet. 2022. PMID: 35709088 Free PMC article.
References
- Alcalai R, Seidman JG, Seidman C. Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J Cardiovasc Electrophysiol. 2008;19:104–110. - PubMed
- Meurs KM, Sanchez X, David RM, Bowles NE, Towbin JA, Reiser PJ, Kittleson JA, Munro MJ, Dryburgh K, MacDonald KA, Kittleson MD. A cardiac myosin binding protein C mutation in the Maine Coon cat with familial hypertrophic cardiomyopathy. Hum Mol Genet. 2005;14:3587–3593. doi: 10.1093/hmg/ddi386. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous