Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated - PubMed (original) (raw)
. 2011 Jun;131(6):1322-30.
doi: 10.103/jid.2011.9. Epub 2011 Feb 10.
Judilyn Fuentes-Duculan, Mayte Suárez-Fariñas, Katherine C Pierson, Alexander Pitts-Kiefer, Linda Fan, Daniel A Belkin, Claire Q F Wang, Shivaprasad Bhuvanendran, Leanne M Johnson-Huang, Mark J Bluth, James G Krueger, Michelle A Lowes, John A Carucci
Affiliations
- PMID: 21307877
- PMCID: PMC3334331
- DOI: 10.103/jid.2011.9
Tumor-associated macrophages in the cutaneous SCC microenvironment are heterogeneously activated
Julia S Pettersen et al. J Invest Dermatol. 2011 Jun.
Abstract
Tumor-associated macrophages (TAMs) may have an important role in tumor immunity. We studied the activation state of TAMs in cutaneous SCC, the second most common human cancer. CD163 was identified as a more abundant, sensitive, and accurate marker of TAMs when compared with CD68. CD163(+) TAMs produced protumoral factors, matrix metalloproteinases 9 and 11 (MMP9 and MMP11), at the gene and protein levels. Gene set enrichment analysis (GSEA) was used to evaluate M1 and M2 macrophage gene sets in the SCC genes and to identify candidate genes in order to phenotypically characterize TAMs. There was coexpression of CD163 and alternatively activated "M2" markers, CD209 and CCL18 (chemokine (C-C motif) ligand 18). There was enrichment for classically activated "M1" genes in SCC, which was confirmed in situ by colocalization of CD163 and phosphorylated STAT1 (signal transducer and activator of transcription 1), IL-23p19, IL-12/IL-23p40, and CD127. Also, a subset of TAMs in SCC was bi-activated as CD163(+) cells expressed markers for both M1 and M2, shown by triple-label immunofluorescence. These data support heterogeneous activation states of TAMs in SCC, and suggest that a dynamic model of macrophage activation would be more useful to characterize TAMs.
Conflict of interest statement
Conflict of Interest
The authors do not have financial interests related to this work.
Figures
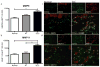
Figure 1. Macrophages were more abundant in SCC compared to normal skin
Representative immunohistochemistry (10x) and cell counts of the macrophage markers (a) CD163 (with an inset of CD163+ cells at 20x) and (b) CD68, showing a significantly increased number of macrophages surrounding SCC tumor nests compared to normal skin. Each dot represents one patient. ***P <0.001. (c) CD163 (green) co-localized with CD68 (red) shown as yellow, but there were CD163+ cells that did not co-express CD68. (d) CD163 (green) did not co-express CD11c (red), while (e) some CD68+ cells (green) did co-express CD11c (red) shown as yellow. Bar=100μm.
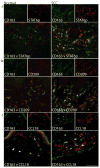
Figure 2. SCC TAMs expressed pro-tumoral products in the tumor microenvironment
Mean mRNA expression of (a) MMP9 and (b) MMP11 relative to HARP after adjustment for batch effect in normal skin (white bars), non-tumoral skin (NT, gray bars), and SCC (black bars) with standard error of the mean. *P <0.05, **P <0.01. CD163+ cells (green) demonstrated abundant co-localization with (c) MMP9 (red) and (d) MMP11 (red) in SCC compared to normal skin. Double positive cells appear yellow. Bar=100μm.
Figure 3. SCC TAMs expressed products of alternatively activated macrophages
Many CD163+ cells (green) co-expressed (a) phosphorylated STAT6 (STAT6p) (red), (b) CD209/DC-SIGN (red), and (c) CCL18 (red) compared to normal skin. Double positive cells appear yellow. Bar=100μm
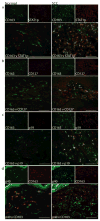
Figure 4. SCC TAMs expressed characteristics of classically activated macrophages in a microenvironment with Type-1 activation
Compared to normal skin, CD163+ cells co-expressed (a) phosphorylated STAT1 (STAT1p) (red), (b) CD127/IL7R (red), (c) IL-23p19 (red), and (d) IL-12/IL-23p40 (green). Double positive cells appear yellow. Bar=100μm
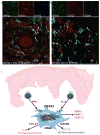
Figure 5. A subset of SCC TAMs simultaneously expressed characteristics of both classical and alternative activation
Triple-labeled confocal immunofluorescence revealed the presence of (a) CD163+ cells (blue) that simultaneously co-expressed STAT6p (green) and STAT1p (red) and (b) CD163+ cells (green) that simultaneously co-expressed the markers CD127 (red) and CD209 (blue) in SCC. Triple-positive cells (white) are indicated by arrows. Bar=100μm. (c) The proposed model of SCC macrophage polarization. Th1 and Th2 cells produce cytokines, IFNγ and IL-4, respectively, and act on resident CD163+ macrophages to polarize these cells in several directions. IFNγ stimulates the M1 phenotype (CD127 and IL-23), and IL-4 stimulates towards the M2 phenotype (CD209 and CCL18). There is also production of mediators that are not driven by known polarizing cytokines, such as MMP9, MMP11, and VEGF-C. The overall outcome is a poly-activated TAM.
Similar articles
- Density and Polarization States of Tumor-Associated Macrophages in Human Cutaneous Squamous Cell Carcinomas Arising in Solid Organ Transplant Recipients.
Cyrus N, Mai-Anh Bui C, Yao X, Kohn LL, Galan A, Rhebergen AM, Colegio OR. Cyrus N, et al. Dermatol Surg. 2016 Jan;42 Suppl 1:S18-23. doi: 10.1097/DSS.0000000000000371. Dermatol Surg. 2016. PMID: 26035047 - Potential role of tumor-associated macrophages and CD163/CD68 ratio in mycosis fungoides and Sézary syndrome in correlation with serum sCD163 and CCL22.
El-Guindy DM, Elgarhy LH, Elkholy RA, Ali DA, Helal DS. El-Guindy DM, et al. J Cutan Pathol. 2022 Mar;49(3):261-273. doi: 10.1111/cup.14155. Epub 2021 Nov 14. J Cutan Pathol. 2022. PMID: 34687561 - Associations between intratumoral and peritumoral M2 macrophage counts and cervical squamous cell carcinoma invasion patterns.
Li Y, Huang G, Zhang S. Li Y, et al. Int J Gynaecol Obstet. 2017 Dec;139(3):346-351. doi: 10.1002/ijgo.12320. Epub 2017 Oct 13. Int J Gynaecol Obstet. 2017. PMID: 28884821 - Prognostic significance of CD68+ and CD163+ tumor associated macrophages in head and neck squamous cell carcinoma: A systematic review and meta-analysis.
Troiano G, Caponio VCA, Adipietro I, Tepedino M, Santoro R, Laino L, Lo Russo L, Cirillo N, Lo Muzio L. Troiano G, et al. Oral Oncol. 2019 Jun;93:66-75. doi: 10.1016/j.oraloncology.2019.04.019. Epub 2019 Apr 28. Oral Oncol. 2019. PMID: 31109698 Review. - Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer.
Tang X. Tang X. Cancer Lett. 2013 May 10;332(1):3-10. doi: 10.1016/j.canlet.2013.01.024. Epub 2013 Jan 21. Cancer Lett. 2013. PMID: 23348699 Review.
Cited by
- Quantitative Evaluation of Macrophage Expression Using CD68 in Oral Submucous Fibrosis: An Immunohistochemical Study.
Pereira T, Naik S, Tamgadge A. Pereira T, et al. Ann Med Health Sci Res. 2015 Nov-Dec;5(6):435-41. doi: 10.4103/2141-9248.177983. Ann Med Health Sci Res. 2015. PMID: 27057383 Free PMC article. - Dealing with Macrophage Plasticity to Address Therapeutic Challenges in Head and Neck Cancers.
Furgiuele S, Descamps G, Cascarano L, Boucq A, Dubois C, Journe F, Saussez S. Furgiuele S, et al. Int J Mol Sci. 2022 Jun 7;23(12):6385. doi: 10.3390/ijms23126385. Int J Mol Sci. 2022. PMID: 35742830 Free PMC article. - The potential role of synovial cells in the progression and treatment of osteoarthritis.
Zou Z, Li H, Yu K, Ma K, Wang Q, Tang J, Liu G, Lim K, Hooper G, Woodfield T, Cui X, Zhang W, Tian K. Zou Z, et al. Exploration (Beijing). 2023 Jul 10;3(5):20220132. doi: 10.1002/EXP.20220132. eCollection 2023 Oct. Exploration (Beijing). 2023. PMID: 37933282 Free PMC article. Review. - Current Perspectives on the Role of Matrix Metalloproteinases in the Pathogenesis of Basal Cell Carcinoma.
Tampa M, Georgescu SR, Mitran MI, Mitran CI, Matei C, Caruntu A, Scheau C, Nicolae I, Matei A, Caruntu C, Constantin C, Neagu M. Tampa M, et al. Biomolecules. 2021 Jun 17;11(6):903. doi: 10.3390/biom11060903. Biomolecules. 2021. PMID: 34204372 Free PMC article. Review. - Regular physical activity prevents chronic pain by altering resident muscle macrophage phenotype and increasing interleukin-10 in mice.
Leung A, Gregory NS, Allen LH, Sluka KA. Leung A, et al. Pain. 2016 Jan;157(1):70-79. doi: 10.1097/j.pain.0000000000000312. Pain. 2016. PMID: 26230740 Free PMC article.
References
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. - PubMed
- Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65. - PubMed
- Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–22. - PubMed
- Bonecchi R, Sozzani S, Stine JT, Luini W, D’Amico G, Allavena P, et al. Divergent effects of interleukin-4 and interferon-gamma on macrophage-derived chemokine production: an amplification circuit of polarized T helper 2 responses. Blood. 1998;92:2668–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL007423-30/HL/NHLBI NIH HHS/United States
- UL1 RR024143-04/RR/NCRR NIH HHS/United States
- UL1 RR024143/RR/NCRR NIH HHS/United States
- K23 AR052404-05/AR/NIAMS NIH HHS/United States
- T32-HL07423/HL/NHLBI NIH HHS/United States
- T32 HL007423/HL/NHLBI NIH HHS/United States
- 1 K23AR052404/AR/NIAMS NIH HHS/United States
- K23 AR052404/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous