Self-renewal versus lineage commitment of embryonic stem cells: protein kinase C signaling shifts the balance - PubMed (original) (raw)
Self-renewal versus lineage commitment of embryonic stem cells: protein kinase C signaling shifts the balance
Debasree Dutta et al. Stem Cells. 2011 Apr.
Abstract
The intricate molecular mechanisms that regulate ESC pluripotency are incompletely understood. Prior research indicated that activation of the Janus kinase-signal transducer and activator of transcription (STAT3) pathway or inhibition of extracellular signal-regulated kinase/glycogen synthase kinase 3 (ERK/GSK3) signaling maintains mouse ESC (mESC) pluripotency. Here, we demonstrate that inhibition of protein kinase C (PKC) isoforms maintains mESC pluripotency without the activation of STAT3 or inhibition of ERK/GSK3 signaling pathways. Our analyses revealed that the atypical PKC isoform, PKCζ plays an important role in inducing lineage commitment in mESCs through a PKCζ-nuclear factor kappa-light-chain-enhancer of activated B cells signaling axis. Furthermore, inhibition of PKC isoforms permits derivation of germline-competent ESCs from mouse blastocysts and also facilitates reprogramming of mouse embryonic fibroblasts toward induced pluripotent stem cells. Our results indicate that PKC signaling is critical to balancing ESC self-renewal and lineage commitment.
Copyright © 2011 AlphaMed Press.
Conflict of interest statement
Conflict of Interest
D.D., S.R. and S.P have filed patent applications through University of Kansas Medical Center. The other authors have no financial interest to disclose.
Figures
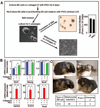
Figure 1. Inhibition of PKC isoform by PKCi supports self-renewal of ES cells
A, Images of E14 ES cells showing undifferentiated colony morphology with PKCi. B, The plot shows percentage of undifferentiated colonies when E14 cells were cultured at clonal density for 5 consecutive passages with PKCi. C, Western blots showing sustained Oct4 expression in PKCi-treated E14 cells. D, E14 cells were maintained with PKCi for higher passages and the images show the undifferentiated colony morphology and nanog expression (inset) in cells of different passages. E, qRT-PCR analysis (mean ± standard error; three independent experiments) of pluripotency gene expression in E14 cells, maintained for different passages with PKCi. Statistically significant (p≤0.05) changes were indicated in the plot. F, E14 cells were cultured at clonal density in N2B27 with PKCi alone. Images show undifferentiated ES cell colony morphology (left) and expression of Oct4 (right) G, qRT–PCR analysis (mean ± standard error; three independent experiments) of Id gene expression in E14 cells, cultured in N2B27 medium with PKCi alone or LIF/BMP4. H, Oct4 staining in E14 cells after five passages (25 days) on collagen IV with PKCi. I, qRT–PCR analysis (mean ± standard error; three independent experiments) of lineage-specific marker expression in E14 cells that were cultured on collagen IV for 5 days with or without PKCi and LIF. Expression of lineage-specific markers was significantly (p≤0.01) inhibited in PKCi-cultred cells compared to cells that were cultured on collagen IV without LIF and PKCi.
Figure 2. Multi-differentiation and developmental potency of ES cells that are maintained at undifferentiated state by inhibiting PKC isoforms
A, Experimental strategy to determine the differentiation potency of ES cells that were cultured in the presence of PKCi for multiple passages. The diagram shows that E14 cells, maintained for 5 passages in PKCi, was differentiated on collagen IV (for 5 days) and generated EBs in the absence of PKCi. The bar graph shows the relative efficiency (mean ± standard error; three independent experiments) of EB formation of PKCi-maintained E14 cells in comparison to cells that were maintained with LIF on plastic. B, Multi-differentiation potency on collagen-IV was determined by measuring mRNA expression (mean ± standard error; three independent experiments) of pluripotency and lineage markers. mRNA levels were measured after culturing on collagen IV for 5 days in the absence and or presence of PKCi. The plot shows significant (p≤0.01) loss in pluripotency gene expression and increase in lineage-specific markers in the absence of PKCi. C, Chimeric mice, generated with E14 cells that were cultured for seven passages (35 days) with PKCi on collagen IV. The table below shows number of chimeras obtained.
Figure 3. De novo derivation of pluripotent ES cells by inhibiting PKC isoforms
A, Upper panels: Blastocyst outgrowth (a) and establishment of ES colony (b) from 129/Sv mice with PKCi. Lower panels: phase (c) and fluorescence (d) images showing Nanog expression in 129/Sv ES cells after four passages with PKCi. B, Chimeric mice produced from PKCi-derived 129/Sv ES cells cultured for 6 passages with PKCi. C, Germline offsprings produced from chimeras that were generated from PKCi-derived 129/Sv ES cells. D, The table shows number of chimeras that produced germline offsprings.
Figure 4. LIF-independent maintenance of self-renewal of PKCζ-depleted ES cells
A, Western blots showing inhibition of phosphorylation of different PKC isoforms by PKCi in E14 cells. B, Images showing differentiation of E14 cells in the presence of selective PKC isoform/s inhibitors. C, Western blots showing loss of RelA(S311) and LGL1/2(S650/S654) phosphorylation in PKCi-treated E14 cells. D, Western blots showing knockdown of PKCζ and loss of RelA(S311) phosphorylation in E14 cells with shRNA molecules, targeted against the 3’ UTR region of PKCζ mRNA. The blots also show rescue of PKCζ expression and RelA phosphorylation with ectopic expression PKCζ from an RNAi immune construct. E, (Top) Images showing maintenance of undifferentiated ES cell colony morphology (left) and expression of Oct4 (right) in PKCζkd cells after five passages without LIF. (Bottom) Images showing rescue of differentiation of PKCζkd cells with ectopic expression of RNAi immune PKCζ. (Inset) Ectopic expression of PKCζ in differentiated cells was confirmed from the expression of EGFP (expressed from the same construct). F, The plot shows percentage of undifferentiated E14 ES cell colonies (mean ± standard error; three independent experiments) when PKCζkd cells, with or without ectopic expression of RNAi-immune PKCζ, were cultured at clonal density. The data indicates significant loss (p≤0.01) of undifferentiated colony formation with ectopic expression of RNAi-immune PKCζ. H, qRT-PCR analysis (mean ± standard error; three independent experiments) showing significant (p≤0.01) loss of pluripotency gene expression in PKCζkd cells upon ectopic expression of PKCζ from the RNAi-immune construct.
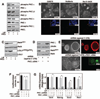
Figure 5. Effect of PKC isoform inhibition on signaling pathways that are implicated in ES cell pluripotency
A, Western blots showing lack of STAT3 (Tyrosine (Y)705) phosphorylation in PKCi-treated E14 ES cells in the absence of LIF. B, Images of Stat3−/− ES cells, cultured with PKCi, showing undifferentiated ES cell colony morphology (left) and expression of Nanog (right). C, Western blot analysis of Akt phosphorylation in E14 ES cells. D, Images showing undifferentiated ES cell colony morphology (left) and expression of Oct4 (right) in E14 ES cells, cultured with PKCi and PI3 kinase inhibitor, Ly294002, in the absence of LIF. E, Western blot analysis of GSK-3phosphorylation in E14 cells when cultured with or without LIF and PKCi. F, Western blot analysis of β-catenin in the cytosolic fractions of E14 cells, cultured with or without LIF, and in the presence of PKCi with or without Wnt3A conditioned medium. G, TopFlash luciferase reporter analysis (mean ± standard error; three independent experiments) in E14 cells, cultured with or without LIF, and in the presence of PKCi with or without Wnt3A conditioned medium (* indicates statistically significant (p≤0.05) changes). H, Western blots showing c-Myc phosphorylation in E14 cells, cultured with or without LIF, and in the presence of PKCi with or without GSK-3 inhibitor CHIR99201. I, Western blots showing ERK1/2 and RSK1 phosphorylation in E14 cells, cultured in the presence and absence of LIF, and in the presence of PKCi with or without ERK inhibitor PD0325901. J, qRT-PCR analysis (mean ± standard error; three independent experiments) of NF-κB target genes (Plaur; plasminogen activator, urokinase receptor, Vim; vimentin, and Igfpb2; insulin-like growth factor binding protein 2) expression in E14 ES cells, cultured with or without LIF and PKCi, and in PKCζkdcells, with or without ectopic expression of RNAi-immune PKCζ cDNA (* indicates statistically significant (p≤0.05) changes). K, Analysis (mean ± standard error; three independent experiments) of NFκ5x-Luc reporter activation in cells, analyzed in panel J. * indicates statistically significant (p≤0.05) change.
Figure 6. PKC inhibition facilitates reprogramming and the derivation of induced pluripotent stem cells
A, Images of a developing iPSC colony from MEFs in the presence of PKCi. B, Immunostaining showing Nanog expression in iPSCs after replating with PKCi at clonal density. C, Chimeric mice that were generated with iPSCs, which are derived and propagated (passage 4) with PKCi. D, The table shows number of chimeras obtained with different iPSC clones. E, Plot shows comparative time course of iPSC colony formation in the presence of LIF and PKCi. F, Relative numbers of iPSC colonies that were derived in different culture conditions (mean ± standard error; three independent experiments, (* indicates statistically significant (p≤0.05) change). G, qRT-PCR analysis (mean ± standard error; three independent experiments) showing significantly (p≤0.05) higher expression of E-Cadherin mRNA and reduced expression of Snail and Slug mRNAs in PKCi-derived iPSCs compared to LIF-derived iPSCs.
Similar articles
- Inhibition of protein kinase C signaling maintains rat embryonic stem cell pluripotency.
Rajendran G, Dutta D, Hong J, Paul A, Saha B, Mahato B, Ray S, Home P, Ganguly A, Weiss ML, Paul S. Rajendran G, et al. J Biol Chem. 2013 Aug 23;288(34):24351-62. doi: 10.1074/jbc.M113.455725. Epub 2013 Jul 11. J Biol Chem. 2013. PMID: 23846691 Free PMC article. - Dual inhibition of Src and GSK3 maintains mouse embryonic stem cells, whose differentiation is mechanically regulated by Src signaling.
Shimizu T, Ueda J, Ho JC, Iwasaki K, Poellinger L, Harada I, Sawada Y. Shimizu T, et al. Stem Cells. 2012 Jul;30(7):1394-404. doi: 10.1002/stem.1119. Stem Cells. 2012. PMID: 22553165 - Signaling pathways dictating pluripotency in embryonic stem cells.
Dutta D. Dutta D. Int J Dev Biol. 2013;57(9-10):667-75. doi: 10.1387/ijdb.130064dd. Int J Dev Biol. 2013. PMID: 24307301 Review. - The Art of Capturing Pluripotency: Creating the Right Culture.
Ying QL, Smith A. Ying QL, et al. Stem Cell Reports. 2017 Jun 6;8(6):1457-1464. doi: 10.1016/j.stemcr.2017.05.020. Stem Cell Reports. 2017. PMID: 28591647 Free PMC article. Review.
Cited by
- Proteomic analysis of early reprogramming events in murine somatic cells incubated with Xenopus laevis oocyte extracts demonstrates network associations with induced pluripotency markers.
Rathbone AJ, Liddell S, Campbell KH. Rathbone AJ, et al. Cell Reprogram. 2013 Aug;15(4):269-80. doi: 10.1089/cell.2012.0083. Epub 2013 Jun 15. Cell Reprogram. 2013. PMID: 23768116 Free PMC article. - Identification of Potential Molecular Determinants of Murine Embryonic Stem Cell Differentiation by a Transposon-Based Approach.
Wang Y, Lei T, Dai Q, Ding P, Qiu T, Fang Y. Wang Y, et al. Mol Biotechnol. 2018 Nov;60(11):791-798. doi: 10.1007/s12033-018-0110-7. Mol Biotechnol. 2018. PMID: 30171517 - Matrix remodeling maintains embryonic stem cell self-renewal by activating Stat3.
Przybyla LM, Theunissen TW, Jaenisch R, Voldman J. Przybyla LM, et al. Stem Cells. 2013 Jun;31(6):1097-106. doi: 10.1002/stem.1360. Stem Cells. 2013. PMID: 23404867 Free PMC article. - Concise Review: Lessons from Naïve Human Pluripotent Cells.
Ware CB. Ware CB. Stem Cells. 2017 Jan;35(1):35-41. doi: 10.1002/stem.2507. Epub 2016 Nov 10. Stem Cells. 2017. PMID: 27663171 Free PMC article. Review.
References
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. - PubMed
- Sato N, Meijer L, Skaltsounis L, et al. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature medicine. 2004;10:55–63. - PubMed
- Ying QL, Nichols J, Chambers I, et al. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL094892/HL/NHLBI NIH HHS/United States
- P20 RR024214/RR/NCRR NIH HHS/United States
- R01 HD062546/HD/NICHD NIH HHS/United States
- R21 HL094892/HL/NHLBI NIH HHS/United States
- P20 RRO24214/PHS HHS/United States
- R21 HD075233/HD/NICHD NIH HHS/United States
- R21 HL104322/HL/NHLBI NIH HHS/United States
- HD062546/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous