Factors associated with acquisition of human infective and animal infective trypanosome infections in domestic livestock in Western Kenya - PubMed (original) (raw)
Factors associated with acquisition of human infective and animal infective trypanosome infections in domestic livestock in Western Kenya
Beatrix von Wissmann et al. PLoS Negl Trop Dis. 2011.
Abstract
Background: Trypanosomiasis is regarded as a constraint on livestock production in Western Kenya where the responsibility for tsetse and trypanosomiasis control has increasingly shifted from the state to the individual livestock owner. To assess the sustainability of these localised control efforts, this study investigates biological and management risk factors associated with trypanosome infections detected by polymerase chain reaction (PCR), in a range of domestic livestock at the local scale in Busia, Kenya. Busia District also remains endemic for human sleeping sickness with sporadic cases of sleeping sickness reported.
Results: In total, trypanosome infections were detected in 11.9% (329) out of the 2773 livestock sampled in Busia District. Multivariable logistic regression revealed that host species and cattle age affected overall trypanosome infection, with significantly increased odds of infection for cattle older than 18 months, and significantly lower odds of infection in pigs and small ruminants. Different grazing and watering management practices did not affect the odds of trypanosome infection, adjusted by host species. Neither anaemia nor condition score significantly affected the odds of trypanosome infection in cattle. Human infective Trypanosoma brucei rhodesiense were detected in 21.5% of animals infected with T. brucei s.l. (29/135) amounting to 1% (29/2773) of all sampled livestock, with significantly higher odds of T. brucei rhodesiense infections in T. brucei s.l. infected pigs (OR = 4.3, 95%CI 1.5-12.0) than in T. brucei s.l. infected cattle or small ruminants.
Conclusions: Although cattle are the dominant reservoir of trypanosome infection it is unlikely that targeted treatment of only visibly diseased cattle will achieve sustainable interruption of transmission for either animal infective or zoonotic human infective trypanosomiasis, since most infections were detected in cattle that did not exhibit classical clinical signs of trypanosomiasis. Pigs were also found to be reservoirs of infection for T. b. rhodesiense and present a risk to local communities.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Figure 1. Map of sampling sites.
A: overview, B: Funyula study villages, C: Butula study villages.
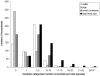
Figure 2. Distribution of household herdsizes.
Number of households per category of herdsize for each host species and for total herdsize.
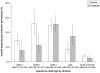
Figure 3. Overall trypanosomiasis prevalence by species and cattle age and division.
Cattle a = cattle under 18 months, cattle b = cattle between 18 and 36 months, cattle c = cattle over 36 months; nB = number of samples from Butula site, nF = number of samples from Funyula site, error bars represent exact binomial 95% confidence interval.
Similar articles
- Impact of mass chemotherapy in domestic livestock for control of zoonotic T. b. rhodesiense human African trypanosomiasis in Eastern Uganda.
Fyfe J, Picozzi K, Waiswa C, Bardosh KL, Welburn SC. Fyfe J, et al. Acta Trop. 2017 Jan;165:216-229. doi: 10.1016/j.actatropica.2016.08.022. Epub 2016 Aug 25. Acta Trop. 2017. PMID: 27570206 - Molecular epidemiological studies on animal trypanosomiases in Ghana.
Nakayima J, Nakao R, Alhassan A, Mahama C, Afakye K, Sugimoto C. Nakayima J, et al. Parasit Vectors. 2012 Oct 1;5:217. doi: 10.1186/1756-3305-5-217. Parasit Vectors. 2012. PMID: 23025330 Free PMC article. - Livestock trypanosomosis in Uganda: parasite heterogeneity and anaemia status of naturally infected cattle, goats and pigs.
Biryomumaisho S, Rwakishaya EK, Melville SE, Cailleau A, Lubega GW. Biryomumaisho S, et al. Parasitol Res. 2013 Apr;112(4):1443-50. doi: 10.1007/s00436-013-3275-9. Epub 2013 Jan 24. Parasitol Res. 2013. PMID: 23344247 - A systematic review and meta-analysis of small ruminant and porcine trypanosomiasis prevalence in sub-Saharan Africa (1986 to 2018).
Ebhodaghe F, Ohiolei JA, Isaac C. Ebhodaghe F, et al. Acta Trop. 2018 Dec;188:118-131. doi: 10.1016/j.actatropica.2018.08.034. Epub 2018 Sep 1. Acta Trop. 2018. PMID: 30179607 Review. - Epidemiology of Trypanosomiasis in Wildlife-Implications for Humans at the Wildlife Interface in Africa.
Kasozi KI, Zirintunda G, Ssempijja F, Buyinza B, Alzahrani KJ, Matama K, Nakimbugwe HN, Alkazmi L, Onanyang D, Bogere P, Ochieng JJ, Islam S, Matovu W, Nalumenya DP, Batiha GE, Osuwat LO, Abdelhamid M, Shen T, Omadang L, Welburn SC. Kasozi KI, et al. Front Vet Sci. 2021 Jun 14;8:621699. doi: 10.3389/fvets.2021.621699. eCollection 2021. Front Vet Sci. 2021. PMID: 34222391 Free PMC article. Review.
Cited by
- Factors associated with persistence of African animal trypanosomiasis in Lango subregion, northern Uganda.
Wangoola RM, Kevin B, Acup CA, Welburn S, Waiswa C, Bugeza J. Wangoola RM, et al. Trop Anim Health Prod. 2019 Sep;51(7):2011-2018. doi: 10.1007/s11250-019-01900-7. Epub 2019 May 3. Trop Anim Health Prod. 2019. PMID: 31054060 - Domestic pigs as potential reservoirs of human and animal trypanosomiasis in Northern Tanzania.
Hamill LC, Kaare MT, Welburn SC, Picozzi K. Hamill LC, et al. Parasit Vectors. 2013 Nov 9;6(1):322. doi: 10.1186/1756-3305-6-322. Parasit Vectors. 2013. PMID: 24499540 Free PMC article. - Molecular identification of trypanosomes in cattle in Malawi using PCR methods and nanopore sequencing: epidemiological implications for the control of human and animal trypanosomiases.
Marsela M, Hayashida K, Nakao R, Chatanga E, Gaithuma AK, Naoko K, Musaya J, Sugimoto C, Yamagishi J. Marsela M, et al. Parasite. 2020;27:46. doi: 10.1051/parasite/2020043. Epub 2020 Jul 20. Parasite. 2020. PMID: 32686644 Free PMC article. - Domestic dogs as reservoirs for African trypanosomiasis in Mambwe district, eastern Zambia.
Lisulo M, Namangala B, Mweempwa C, Banda M, Chambaro H, Moonga L, Kyoko H, Chihiro S, Picozzi K, Maciver SK, MacLeod ET. Lisulo M, et al. Sci Rep. 2024 Sep 10;14(1):21062. doi: 10.1038/s41598-024-69834-1. Sci Rep. 2024. PMID: 39256442 Free PMC article. - Prevalence and Associated Risk Factors of African Animal Trypanosomiasis in Cattle in Lambwe, Kenya.
Okello I, Mafie E, Eastwood G, Nzalawahe J, Mboera LEG, Onyoyo S. Okello I, et al. J Parasitol Res. 2022 Jul 14;2022:5984376. doi: 10.1155/2022/5984376. eCollection 2022. J Parasitol Res. 2022. PMID: 35872666 Free PMC article.
References
- Kristjanson PM, Swallow BM, Rowlands GJ, Kruska RL, de Leeuw PN. Measuring the costs of African animal trypanosomosis, the potential benefits of control and returns to research. Agricultural Systems. 1999;59:79–98.
- WHO. Human African Trypanosomiasis (sleeping sickness): epidemiological update. Weekly epidemiological record. 2006;81:71–80. - PubMed
- Government of Kenya. Nairobi: Ministry of Finance and Planning; 2001. Busia District Development Plan 2002–2008. Effective management for sustainable economic growth and poverty reduction.
- FITCA. Farming in Tsetse Controlled Areas. 2005. Kenya Project 1999–2006. Lessons Learned.
- Taylor K, Authié E. Pathogenesis of animal trypanosomiasis. In: Maudlin I, Holmes PH, Miles MA, editors. The Trypanosomiases. Wallingford, Oxfordshire: CABI Publishing; 2004. pp. 331–353.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources