Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM-dependent mechanism - PubMed (original) (raw)
Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM-dependent mechanism
Gerald J Atkins et al. J Bone Miner Res. 2011 Jul.
Abstract
The identity of the cell type responsive to sclerostin, a negative regulator of bone mass, is unknown. Since sclerostin is expressed in vivo by mineral-embedded osteocytes, we tested the hypothesis that sclerostin would regulate the behavior of cells actively involved in mineralization in adult bone, the preosteocyte. Differentiating cultures of human primary osteoblasts exposed to recombinant human sclerostin (rhSCL) for 35 days displayed dose- and time-dependent inhibition of in vitro mineralization, with late cultures being most responsive in terms of mineralization and gene expression. Treatment of advanced (day 35) cultures with rhSCL markedly increased the expression of the preosteocyte marker E11 and decreased the expression of mature markers DMP1 and SOST. Concomitantly, matrix extracellular phosphoglycoprotein (MEPE) expression was increased by rhSCL at both the mRNA and protein levels, whereas PHEX was decreased, implying regulation through the MEPE-ASARM axis. We confirmed that mineralization by human osteoblasts is exquisitely sensitive to the triphosphorylated ASARM-PO4 peptide. Immunostaining revealed that rhSCL increased the endogenous levels of MEPE-ASARM. Importantly, antibody-mediated neutralization of endogenous MEPE-ASARM antagonized the effect of rhSCL on mineralization, as did the PHEX synthetic peptide SPR4. Finally, we found elevated Sost mRNA expression in the long bones of HYP mice, suggesting that sclerostin may drive the increased MEPE-ASARM levels and mineralization defect in this genotype. Our results suggest that sclerostin acts through regulation of the PHEX/MEPE axis at the preosteocyte stage and serves as a master regulator of physiologic bone mineralization, consistent with its localization in vivo and its established role in the inhibition of bone formation.
Copyright © 2011 American Society for Bone and Mineral Research.
Conflict of interest statement
Disclosures
All authors state that they have no conflicts of interest.
Figures
Fig. 1
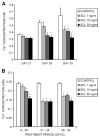
Effects of exogenously added SCL on NHBC in vitro mineralization. NHBCs were cultured under mineralizing conditions for up to 35 days in the absence or presence of rhSCL at 1, 10, or 50 ng/mL. The medium was replenished every 3 to 4 days. (A) Continuously treated cultures were assayed on days 21, 28, and 35 for cell layer–associated calcium levels, determined as described in “Materials and Methods.” (B) Comparison of in vitro mineralization when cells were treated with rhSCL for the entire 35-day period, from days 21 to 35, or from days 28 to 35. Note that NHBCs from different donors were used for the two experiments depicted. Data represent mean values from quadruplicate wells ± SD. *Difference Difference from control (no added rhSCL), p <.05.
Fig. 2
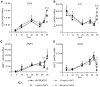
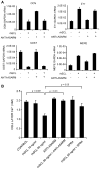
Effects on gene expression of continuous exposure of mineralizing NHBC cultures to exogenously added SCL. NHBCs were cultured under mineralizing conditions for up to 35 days in the absence or presence of rhSCL at 1, 10, or 50 ng/mL. The medium was replenished every 3 to 4 days. At the time points indicated, total RNA was prepared and real-time RT-PCR was performed to determine mRNA expression of (A) OCN, (B) E11, (C) DMP1, and (D) SOST. Data shown are means of triplicate reactions ± SD normalized to expression of GAPDH mRNA. a, b, and c indicate significant differences (p <.05) from untreated controls for rhSCL at 1, 10, and 50 ng/mL, respectively. Similar results were obtained from three independent experiments using NHBCs from different donors.
Fig. 3
Effects on gene expression of acute exposure of mature NHBC cultures to exogenously added SCL. NHBCs were cultured under mineralizing conditions for 35 days. Cells then were cultured for a further 3 or 7 days in the absence or presence of rhSCL at 1, 10, or 50 ng/mL. The medium and supplements were replenished on day 3. Total RNA was prepared and real-time RT-PCR was performed to determine mRNA expression of (A) OCN, (B) COL1α1, (C) E11, (D) TNAP, (E) DMP1, (F) SOST, (G) LRP4, (H) PHEX, and (I) MEPE. Data shown are means of triplicate reactions ± SD normalized to expression of GAPDH mRNA. a, b, and c indicate significant differences (p <.05) from untreated controls for rhSCL at 1, 10, and 50 ng/mL, respectively. Near-identical results were obtained from three independent experiments using NHBCs from different donors.
Fig. 4
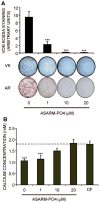
Effect on in vitro mineralization of NHBCs of ASARM-PO4. NHBCs were cultured under mineralizing conditions for 28 days in the absence or presence of ASARM-PO4 peptide at 1, 10, or 20 μM final concentration. (A) Cell layers were assayed for phosphate incorporation by Von Kossa (VK) staining or calcium incorporation using alizarin red (AR) staining. VK stains were quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Data are means of quadruplicate cultures ± SD. ***Difference from untreated control (p <.001). (B) Supernatant or cell-free (CF) medium taken from the day 28 time point (representing an incubation period of 3 days) was assayed for remaining calcium concentration, as described in “Materials and Methods,” and expressed as a ratio to the level present in the CF control. Data are means of quadruplicate cultures ± SD. ***Difference from CF control (p <.001).
Fig. 5
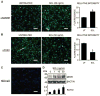
The effect of rhSCL on endogenous levels of MEPE-ASARM. NHBCs were cultured under mineralizing conditions for 35 days in chamber slides and then treated for a further 3 days in the absence or presence of rhSCL (50 ng/mL). Cells were stained by immunofluorescence antibodies for (A) MEPE-ASARM and (B) PHEX and analyzed by confocal microscopy. Representative images of triplicate wells are shown for each treatment. The relative mean fluorescence intensity ± SEM (arbitrary units) of the cell layer for each treatment was quantified using ImageJ analysis of images (n =6) taken of the unmerged antibody-specific (FITC) signal. (C) The level of background staining using a normal rabbit IgG as the primary antibody. Nuclei were stained using DAPI, as described in “Materials and Methods.” (D) The effect of a 3-day treatment of day 35 differentiated NHBC cultures with rhSCL (0 to 50 ng/mL) on full-length MEPE expression assessed by Western blot. Relative MEPE levels were compared with those of β-actin, which served as a loading control, and represent four independent blots. In all cases, an asterisk indicates difference from untreated (p <.05).
Fig. 6
The effect of neutralizing antibody to ASARM-PO4 on the response to SCL. (A) NHBCs cultured under mineralizing conditions for 35 days then were cultured either untreated (control) or with rhSCL at 50 ng/mL, neutralizing antibody to ASARM-PO4 (10 μg/mL), or a combination for 3 days, and the effect on gene expression was measured for OCN, E11, SOST, and MEPE. Data are means of triplicate reactions ± SD and are representative of two independent experiments. (B) The inhibitory effect of SCL on mineralization is reversed by ASARM-PO4 and SPR4 peptides. NHBCs were cultured under mineralizing conditions for 35 days as earlier and then were cultured for a further 7 days either untreated (control) or with combinations of rhSCL at 10 and 50 ng/mL, neutralizing antibody to ASARM-PO4 (10 μg/mL), or SPR4 peptide (10 μM). The medium and supplements were replenished on day 3. Cell layer–associated calcium was determined after 7 days, as described in “Materials and Methods.” Data are expressed as means ± SD of quadruplicate observations. Significance, as indicated, was determined by one-way ANOVA. Similar results were obtained in four independent experiments.
Fig. 7
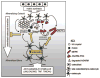
Cartoon representing the proposed effects of SCL as a master regulator of bone mineralization. Anti-anabolic stimuli, such as mechanical unloading or proinflammatory cytokines TNF-α and TWEAK, induce the expression of SCL by mineral-embedded osteocytes. As indicated (yellow arrow), SCL then acts on late osteoblasts/preosteocytes in local osteoid by stimulating the expression of phosphorylated MEPE, which is cleaved (by cathepsin B) into phosphorylated ASARM peptides (ASARM-PO4). ASARM-PO4 peptides attach to nascent hydroxyapatite-like (HA) crystals and prevent further mineralization. PHEX promotes mineralization by inhibiting MEPE cleavage into ASARM peptides and by degrading mineralization-inhibiting ASARM-PO4 peptides, reversing the inhibitory effect of MEPE on mineralization. SCL decreases PHEX expression (yellow bar), potentially by either late osteoblasts through to mature osteocytes, thereby decreasing this positive effect. The net inhibition of mineralization by SCL is accompanied by inhibition of late osteoblast/preosteocyte transition into mature osteocytes (yellow bar), as described in the text.
Similar articles
- Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP.
Martin A, David V, Laurence JS, Schwarz PM, Lafer EM, Hedge AM, Rowe PS. Martin A, et al. Endocrinology. 2008 Apr;149(4):1757-72. doi: 10.1210/en.2007-1205. Epub 2007 Dec 27. Endocrinology. 2008. PMID: 18162525 Free PMC article. - Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway.
Rowe PS. Rowe PS. Crit Rev Eukaryot Gene Expr. 2012;22(1):61-86. doi: 10.1615/critreveukargeneexpr.v22.i1.50. Crit Rev Eukaryot Gene Expr. 2012. PMID: 22339660 Free PMC article. Review. - MEPE-ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite: an inhibition regulated by PHEX cleavage of ASARM.
Addison WN, Nakano Y, Loisel T, Crine P, McKee MD. Addison WN, et al. J Bone Miner Res. 2008 Oct;23(10):1638-49. doi: 10.1359/jbmr.080601. J Bone Miner Res. 2008. PMID: 18597632 - SPR4-peptide alters bone metabolism of normal and HYP mice.
Zelenchuk LV, Hedge AM, Rowe PS. Zelenchuk LV, et al. Bone. 2015 Mar;72:23-33. doi: 10.1016/j.bone.2014.11.011. Epub 2014 Nov 22. Bone. 2015. PMID: 25460577 Free PMC article. - FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization.
Quarles LD. Quarles LD. Am J Physiol Endocrinol Metab. 2003 Jul;285(1):E1-9. doi: 10.1152/ajpendo.00016.2003. Am J Physiol Endocrinol Metab. 2003. PMID: 12791601 Review.
Cited by
- Age dependent regulation of bone-mass and renal function by the MEPE ASARM-motif.
Zelenchuk LV, Hedge AM, Rowe PS. Zelenchuk LV, et al. Bone. 2015 Oct;79:131-42. doi: 10.1016/j.bone.2015.05.030. Epub 2015 Jun 4. Bone. 2015. PMID: 26051469 Free PMC article. - DNA methylation regulates sclerostin (SOST) expression in osteoarthritic chondrocytes by bone morphogenetic protein 2 (BMP-2) induced changes in Smads binding affinity to the CpG region of SOST promoter.
Papathanasiou I, Kostopoulou F, Malizos KN, Tsezou A. Papathanasiou I, et al. Arthritis Res Ther. 2015 Jun 12;17(1):160. doi: 10.1186/s13075-015-0674-6. Arthritis Res Ther. 2015. PMID: 26071314 Free PMC article. - Fibroblast Growth Factor 2 and Its Receptors in Bone Biology and Disease.
Coffin JD, Homer-Bouthiette C, Hurley MM. Coffin JD, et al. J Endocr Soc. 2018 May 28;2(7):657-671. doi: 10.1210/js.2018-00105. eCollection 2018 Jul 1. J Endocr Soc. 2018. PMID: 29942929 Free PMC article. Review. - Osteocyte Dysfunction in Joint Homeostasis and Osteoarthritis.
Zhang L, Wen C. Zhang L, et al. Int J Mol Sci. 2021 Jun 17;22(12):6522. doi: 10.3390/ijms22126522. Int J Mol Sci. 2021. PMID: 34204587 Free PMC article. Review. - Histological evidence that metformin reverses the adverse effects of diabetes on orthodontic tooth movement in rats.
Sun J, Du J, Feng W, Lu B, Liu H, Guo J, Amizuka N, Li M. Sun J, et al. J Mol Histol. 2017 Apr;48(2):73-81. doi: 10.1007/s10735-016-9707-y. Epub 2016 Dec 15. J Mol Histol. 2017. PMID: 27981392
References
- Baron R, Rawadi G, Roman-Roman S. Wnt signaling: a key regulator of bone mass. Curr Top Dev Biol. 2006;76:103–127. - PubMed
- Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–869. - PubMed
- ten Dijke P, Krause C, de Gorter DJ, Lowik CW, van Bezooijen RL. Osteocyte-derived sclerostin inhibits bone formation: its role in bone morphogenetic protein and Wnt signaling. J Bone Joint Surg Am. 2008;90:S31–35. - PubMed
- Staehling-Hampton K, Proll S, Paeper BW, et al. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am J Med Genet. 2002;110:144–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases