DSL-Notch signaling in the Drosophila brain in response to olfactory stimulation - PubMed (original) (raw)
Comparative Study
DSL-Notch signaling in the Drosophila brain in response to olfactory stimulation
Toby Lieber et al. Neuron. 2011.
Abstract
Delta/Serrate/Lag2 (DSL) ligands and their Notch family receptors have profound and pervasive roles in development. They are also expressed in adult tissues, notably in mature neurons and glia in the brain, where their roles are unknown. Here, focusing on the sense of smell in adult Drosophila, we show that Notch is activated in select olfactory receptor neurons (ORNs) in an odorant-specific fashion. This response requires olfactory receptor activity and the Notch ligand Delta. We present evidence that Notch activation depends on synaptic transmission by the ORNs in which the receptors are active and is modulated by the activity of local interneurons in the antennal lobe. It is also subject to regulatory inputs from olfactory receptor activity in other ORNs. These findings identify a correlate of stimulus-dependent brain activity and potentially new forms of neural integration and plasticity.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
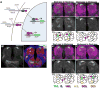
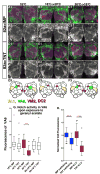
Figure 1. The olfactory environment modulates the pattern of Notch activation
A. N-GV and N-LV assays for Notch activation. Binding of Delta to the ectodomain of Notch-GAL4-VP16 (N-GV, upper) and Notch-LexA-VP16 (N-LV, lower) proteins induces juxtamembrane (S2) followed by intramembrane (S3) cleavages, releasing GAL4-VP16 and LexA-VP16 from the membrane, allowing them to activate transcription from UAS.dGFP and LexOP.dGFP reporters in the nucleus. dGFP encodes a destabilized form of GFP. See also Figs. S1A, B. B. Visualization of Notch activity in the adult brain using the N-GV system. N-GV UAS.dGFP/+ (“NGV>dGFP”) flies were raised on cornmeal food supplemented with molasses as a sugar source. dGFP can be detected in the D glomerulus in the antennal lobe (AL), in the antennal nerve (AN) and in neurons that project to the subesophageal ganglion (SOG). Here, and in all of the remaining figures, glomerular morphology has been visualized using nc82 antisera directed against Bruchpilot, a presynaptic marker (Wagh et al., 2006). Glomeruli were identified using the maps of Stocker et al., 1990 and Couto et al., 2005. Neuronal cell bodies are stained with anti-ELAV. AL neurons mediate olfaction, gustatory neurons project to the SOG (Wang et al., 2004b) and Johnston’s organ neurons project through the AN to the antennomechanosensory center (Sivan-Loukianova and Eberl, 2005). See also Fig. S1C. C–F. N-GV UAS.dGFP/+ flies raised to eclosion and then aged for four days on cornmeal food supplemented with dextrose or molasses as a sugar source (hence forth “dextrose” or “molasses” food) were transferred for four days to food with the same sugar source (C & D) or to standard food with the other sugar source (E & F). The pattern of dGFP accumulation in response to dextrose food differs from that in response to molasses; changing the food source causes a corresponding change in the dGFP response. Here, and in subsequent figures, the results are also depicted in cartoon form using a standard diagram of left and right antennal lobes, with relevant glomeruli indicated by color (as in the color key): solid coloring indicates a positive dGFP response, and colored dotted outlines indicates a negative response. Some glomeruli (e.g., DC3 in this panel), are located beneath the plane of focus shown in the cartoon, and hence do not correspond with the black outlines depicting the morphology of the glomeruli located at the surface of the antennal lobe.
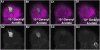
Figure 2. Notch is activated in response to defined odors
N-GV/UAS.dGFP flies raised to eclosion and aged for four days on dextrose food were exposed for four days to different concentrations of geranyl acetate diluted in paraffin oil (A–D), or to atmospheric CO2 (0.03%) (E) or 1% CO2 for three days (F). Exposure to geranyl acetate results in dGFP accumulation predominantly in the VA6 glomerulus and exposure to CO2 results in predominant dGFP accumulation the V glomerulus. All brains in A–D were processed at the same time and imaged at the same microscope settings. To ensure that the geranyl acetate signal was in the linear range, the settings were such that the Notch food response is not visible.
Figure 2. Notch is activated in response to defined odors
N-GV/UAS.dGFP flies raised to eclosion and aged for four days on dextrose food were exposed for four days to different concentrations of geranyl acetate diluted in paraffin oil (A–D), or to atmospheric CO2 (0.03%) (E) or 1% CO2 for three days (F). Exposure to geranyl acetate results in dGFP accumulation predominantly in the VA6 glomerulus and exposure to CO2 results in predominant dGFP accumulation the V glomerulus. All brains in A–D were processed at the same time and imaged at the same microscope settings. To ensure that the geranyl acetate signal was in the linear range, the settings were such that the Notch food response is not visible.
Figure 3. Kinetics of Notch activation in response to increasing or decreasing exposure to CO2
A,B. N-GV/Gr21a.dsRed; UAS.GFP/+ flies were raised to eclosion and aged on dextrose food for four to seven days, then shifted to agar and exposed to 5–10% CO2 for the indicated time periods. The nuclear GFP (green) and dsRed (magenta) fluorescence were visualized in whole mount antennae (coincident fluorescence appears white); note that some non-Gr21a expressing ORNs have accumulated GFP, presumably in response to other odorants (A). The depicted 72hr antenna was exposed to 7% CO2. All other images of CO2 exposed antennae are from flies exposed to 5% CO2. The graph (B) depicts box plots of the % Gr21a.dsRed cells that expressed GFP at the indicated times of exposure and % CO2 (in parentheses). Here and in all box plots presented below, the horizontal line in the box is the median of the entire data set; the lines at the top and bottom of the box are the medians of the higher and lower 50% respectively; the whiskers at the top and bottom are the maximum and minimum values excluding the outliers (dots), which are more than 3/2 times the upper quartile or less than 3/2 times the lower quartile. The Mann-Whitney test was applied to data from pairs of time points. ns: not significant; *: P≤ 0.05; **: P≤ 0.01; ***: P≤ 0.001. The number of samples analyzed was: 0hrs, 17; 6hrs (5%), 12; 6hrs (10%), 19; 12hrs (5%), 11; 18hrs (5%), 14; 24hrs (5%), 18; 24hrs (7%), 19; 72hrs (7%), 13. C. As in A, except that N-GV UAS.dGFP/+ flies were used, and exposed to 5% CO2 on dextrose food for 3 days. Flies were removed from CO2 for the indicated lengths of time, and the Notch response assayed by quantitating dGFP accumulation in the V glomerulus. The graph depicts box plots of the integrated density of fluorescence in V. The number of samples analyzed was: 0 days, 32; 1day, 28; 2 days, 28; 3 days, 23; 4 days, 26; no CO2, 17.
Figure 4. Notch activation in response to odor is Delta dependent
A–D. N-GV UAS.dGFP/+ flies that are genotypically +/+, DlRF/+, Dl6B/+, or DlRF/Dl6B, were raised at 18°C on molasses food and shifted to dextrose food upon eclosion. Flies were aged at 18°C for 5–6 days and then shifted to 30° C for one day prior to exposure to 5% CO2 on agar at 30° C for three days. The dGFP response is reduced progressively in the weaker DlRF/+ and stronger Dl6B/+ genotypes, and is absent in DlRF/Dl6B transheterozygotes. See also Fig. S2. E–H. N-GV UAS.dGFP/+ flies that are genotypically +/+ (E,F) or DlRF/Dl6B (G,H) were raised and aged as in A–D. Flies were then shifted to 30°C for 4 days, shifted back to 18°C for one day and exposed to 5% CO2 on agar at 18°C for 3 days. The dGFP response is restored upon return to the permissive temperature. I. The graph presents an experiment analogous to that shown in A–D, except the flies were exposed to a 1:1000 dilution of geranyl acetate, rather than 5% CO2. The box plots depict dGFP accumulation in the VA6 glomerulus. The results are the same as for CO2, except that there is no significant decline in the geranyl acetate response in the weaker DlRF/+ genotype. The number of samples analyzed was: wt, 20; DlRF/+, 22; Dl6B/+, 14; DlRF/Dl6B, 16.
Figure 5. Notch activation in response to odor requires odorant receptor
A,B. wild type (A) and Or43b− (B) flies, that are also N-GV/UAS.dGFP, were raised and maintained on dextrose food for three to five days and then exposed to a 1:100 dilution of ethyl butyrate for 4 days. Note the absence of the ethyl butyrate response in VM2 in Or43b mutants, as well as the increase in dGFP accumulation in VA6, DM5, DM6 and D compared to the wild type control. C. Or43b− flies (C) flies, that are also N-GV/UAS.dGFP, were exposed to a 1:100 dilution of geranyl acetate. Predominant dGFP accumulation is in VA6, as in control (wild type) flies (Fig. 2A). D–F. Or43b− flies carrying the Or43b.Or82a transgene (Or43b−; 43b>Or82a), as well as N-GV and UAS.dGFP, were exposed to paraffin oil (D), or to 1:100 dilutions of ethyl butyrate (E) or geranyl acetate (F). Ectopic expression of the Or82a receptor in Or43b ORNs innervating VM2 is associated with an ectopic dGFP response to geranyl acetate in VM2 (F), but no rescue of dGFP accumulation in VM2 in response to ethyl butyrate (E). Similarly, it is associated with the loss of the prominent dGFP response seen in VA6 and DM6 in Or43b mutant (B), but not wild type flies (A).
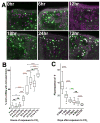
Figure 6. Notch activation in odorant receptor neurons depends on synaptic transmission
A–F. tubP-gal80ts20; N-LV LexOP.dGFP flies carrying an Or82a.Gal4 driver, and either the UAS.IMPTNT-Q4A (IMP; A–C) or UAS.TNT-E (TNT; D–F) transgene, as indicated, were raised at 18°C on molasses food and shifted to dextrose food upon eclosion. The 18°C set (A and D) were aged at 18°C for 11 days and then exposed to a 1:100 dilution of geranyl acetate oil for 4 days at 18°C. The 18°C->30°C set (B and E) were aged at 18°C for 4 days and shifted to 30°C for 4 days prior to being exposed to exposed to geranyl acetate at 30°C for an additional 4 days. The 30°C->18°C set (C and F) were aged at 18°C for 2 days, shifted to 30°C for 4 days, shifted back to 18°C for 4 days and then exposed to geranyl acetate for 4 days at 18°. As depicted in D3, E3 and F3, expression of tetanus toxin (TNT) for eight days at 30°C significantly reduces dGFP accumulation in VA6 in response to geranyl acetate relative to control flies expressing inactive toxin (IMP) under the same conditions. Moreover, the effect is reversible following return to 18° C, which blocks expression of the toxin. (The phenotype we see cannot be due to a direct effect of TNT on Notch, because while Notch is required throughout development, TNT expression has been shown not to affect embryonic development (Sweeney et al., 1995).) G. The graph depicts box plots of the integrated density of fluorescence in VA6. Only the 30°C set differed significantly. The number of samples analyzed was: 18°C IMP, 22; 18°C TNT, 24; 30°C IMP, 22; 30°C TNT, 24; 30>18°C IMP, 22; 30>18°C TNT, 28. H. The graph depicts box plots of the integrated density of fluorescence in VA6 normalized to the IMP control in each data set. N-LV IMP (IMP N-LV) and N-LV TNT (TNT N-LV) flies of the same genotype as in A-F and dGFP/tubP-gal80ts20 UAS.IMPTNT-Q4A; 82a GAL4/+ (IMP trafficking) and dGFP/tubP-gal80ts20 UAS.TNT-E; 82a GAL4/+ (TNT trafficking) flies were raised at 18°C on molasses food and shifted to dextrose food upon eclosion. They were aged at 18°C for 4 days and shifted to 30°C for one day prior to being exposed to a 1:100 dilution of geranyl acetate for 4 days. While both the reduction in Notch activity and dGFP trafficking are significant, the reduction in Notch activity is greater (61%) than the reduction in dGFP trafficking (26%).
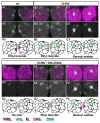
Figure 7. Notch activation in olfactory receptor neurons is modulated by synaptic activity of local interneurons in the antennal lobe
N-LV LexOP.dGFP/tubP-gal80ts20 flies carrying Kra.Gal4, LN1.Gal4, or LN2.Gal4 drivers and either the UAS.IMPTNT-Q4-A (IMP) or UAS.TNT-E (TNT) transgene, as indicated, were raised to eclosion on molasses food and aged for two to four days on dextrose food at 18° C. They were then transferred to 30°C for four days and exposed at 30°C to a 1:100 dilution of geranyl acetate for four more days or to 5% CO2 (Kra.GAL4 and LN2.GAL4) or 10% CO2 (LN1.GAL4) for three more days, as indicated. TNT expression under the control of each of the three LN drivers significantly altered the level of dGFP accumulation in response to either, or both, geranyl acetate and CO2, in the appropriate target glomerulus (VA6 for geranyl acetate and V for CO2). In the cartoons, darker shades of green indicate increased Notch activity. For each set of experiments, the graphs depict box plots of fluorescence in VA6 (geranyl acetate exposed flies) or V (CO2 exposed flies). The number of samples analyzed for each genotype and odor was: Kra>IMP geranyl acetate, 14; Kra>TNT geranyl acetate, 26; Kra>IMP CO2, 33; Kra>TNT CO2, 27; LN1>IMP geranyl acetate, 25; LN1>TNT geranyl acetate, 28; LN1>IMP CO2, 41; LN1>TNT CO2, 33; LN2>IMP geranyl acetate, 30; LN2>TNT geranyl acetate, 26; LN2>IMP CO2, 32; LN2>TNT CO2, 24.
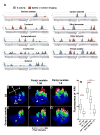
Figure 8. Notch activation induced by odorant mixtures depends on inhibitory cross talk between responding olfactory receptor neurons
A. 4 day old N-GV UAS.dGFP/+ flies which had been raised on dextrose food were exposed to 1:100 dilutions of odors (for 4 days) or to 5% CO2 (for 3 days). Seven to twelve flies were assayed for each odor. The fraction of flies with Notch activity in the indicated glomeruli is presented in blue. The activity of the ORNs in response to puffs of the corresponding odor is shown in red. With the exception of 3-octanol this is electrophysiological data (de Bruyne et al., 2001; Hallem and Carlson, 2006) and the height of the red bars reflects spike number. Glomeruli activated by 3-octanol were determined by calcium imaging (Wang et al., 2003). No obvious changes in Notch activation were observed for the following odors: phenethyl acetate, methyl salicylate, cis-vaccenyl acetate, octyl aldehyde. B–H. N-GV UAS.dGFP/+ flies raised to eclosion and aged for four days on dextrose food were exposed for four days to different concentrations of geranyl acetate and pentyl acetate as indicated, or to paraffin oil alone. Surface plots of Notch activation in an antennal lobe are depicted in B–G, with the height and color of the peaks being proportional to pixel intensity. Orientation of the antennal lobe is depicted in B: M, medial; D, dorsal; L, lateral; V, ventral. Addition of pentyl acetate to geranyl acetate at concentrations of 1:50 and 1:5 caused stepwise reductions in dGFP accumulation in VA6 (asterisk). Conversely, there is an increase in dGFP accumulation in VA3 (arrowhead). Box plots of the fluorescence in VA6 are presented in H, illustrating the reduction in Notch activation in VA6 induced by pentyl acetate (GA, geranyl acetate; PA, pentyl acetate). The number of samples analyzed was: oil, 24; PA 1:50, 24; GA 1:1000, 32; GA 1:1000 PA 1:5, 32; GA 1:1000 PA 1:50, 30.
Similar articles
- Notch is required in adult Drosophila sensory neurons for morphological and functional plasticity of the olfactory circuit.
Kidd S, Struhl G, Lieber T. Kidd S, et al. PLoS Genet. 2015 May 26;11(5):e1005244. doi: 10.1371/journal.pgen.1005244. eCollection 2015 May. PLoS Genet. 2015. PMID: 26011623 Free PMC article. - Mechanism of Notch Pathway Activation and Its Role in the Regulation of Olfactory Plasticity in Drosophila melanogaster.
Kidd S, Lieber T. Kidd S, et al. PLoS One. 2016 Mar 17;11(3):e0151279. doi: 10.1371/journal.pone.0151279. eCollection 2016. PLoS One. 2016. PMID: 26986723 Free PMC article. - Farnesol-detecting olfactory neurons in Drosophila.
Ronderos DS, Lin CC, Potter CJ, Smith DP. Ronderos DS, et al. J Neurosci. 2014 Mar 12;34(11):3959-68. doi: 10.1523/JNEUROSCI.4582-13.2014. J Neurosci. 2014. PMID: 24623773 Free PMC article. - [Notch signal diversifies olfactory receptor neurons and determines their axonal projection pattern in Drosophila melanogaster].
Endo K, Hama C. Endo K, et al. Tanpakushitsu Kakusan Koso. 2007 Sep;52(11):1330-6. Tanpakushitsu Kakusan Koso. 2007. PMID: 17867287 Review. Japanese. No abstract available. - Olfactory information processing in Drosophila.
Masse NY, Turner GC, Jefferis GS. Masse NY, et al. Curr Biol. 2009 Aug 25;19(16):R700-13. doi: 10.1016/j.cub.2009.06.026. Curr Biol. 2009. PMID: 19706282 Review.
Cited by
- Olfactory Critical Periods: How Odor Exposure Shapes the Developing Brain in Mice and Flies.
Mallick A, Dacks AM, Gaudry Q. Mallick A, et al. Biology (Basel). 2024 Feb 2;13(2):94. doi: 10.3390/biology13020094. Biology (Basel). 2024. PMID: 38392312 Free PMC article. Review. - Glomerulus-Selective Regulation of a Critical Period for Interneuron Plasticity in the Drosophila Antennal Lobe.
Chodankar A, Sadanandappa MK, VijayRaghavan K, Ramaswami M. Chodankar A, et al. J Neurosci. 2020 Jul 15;40(29):5549-5560. doi: 10.1523/JNEUROSCI.2192-19.2020. Epub 2020 Jun 12. J Neurosci. 2020. PMID: 32532889 Free PMC article. - Notch signaling and neural connectivity.
Giniger E. Giniger E. Curr Opin Genet Dev. 2012 Aug;22(4):339-46. doi: 10.1016/j.gde.2012.04.003. Epub 2012 May 17. Curr Opin Genet Dev. 2012. PMID: 22608692 Free PMC article. Review. - Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila.
Liu W, Liang X, Gong J, Yang Z, Zhang YH, Zhang JX, Rao Y. Liu W, et al. Nat Neurosci. 2011 Jun 19;14(7):896-902. doi: 10.1038/nn.2836. Nat Neurosci. 2011. PMID: 21685916 - Lunatic Fringe-GFP Marks Lamina-Specific Astrocytes That Regulate Sensory Processing.
Akdemir ES, Woo J, Bosquez Huerta NA, Lozzi B, Groves AK, Harmanci AS, Deneen B. Akdemir ES, et al. J Neurosci. 2022 Jan 26;42(4):567-580. doi: 10.1523/JNEUROSCI.1392-21.2021. Epub 2021 Dec 6. J Neurosci. 2022. PMID: 34872929 Free PMC article.
References
- Alfonso TB, Jones BW. gcm2 promotes glial cell differentiation and is required with glial cells missing for macrophage development in Drosophila. Dev Biol. 2002;248:369–383. - PubMed
- Barolo S, Carver LA, Posakony JW. GFP and beta-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques. 2000;29:726, 728, 730, 732. - PubMed
- Barolo S, Castro B, Posakony JW. New Drosophila transgenic reporters: insulated P-element vectors expressing fast-maturing RFP. Biotechniques. 2004;36:436–440. 442. - PubMed
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. - PubMed
- Berezovska O, McLean P, Knowles R, Frosh M, Lu FM, Lux SE, Hyman BT. Notch1 inhibits neurite outgrowth in postmitotic primary neurons. Neuroscience. 1999;93:433–439. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases