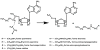Current status of the polyamine research field - PubMed (original) (raw)
Review
Current status of the polyamine research field
Anthony E Pegg et al. Methods Mol Biol. 2011.
Abstract
This chapter provides an overview of the polyamine field and introduces the 32 other chapters that make up this volume. These chapters provide a wide range of methods, advice, and background relevant to studies of the function of polyamines, the regulation of their content, their role in disease, and the therapeutic potential of drugs targeting polyamine content and function. The methodology provided in this new volume will enable laboratories already working in this area to expand their experimental techniques and facilitate the entry of additional workers into this rapidly expanding field.
Figures
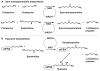
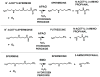
Fig 1
Structures of some naturally occurring polyamines. It should be noted that at physiological pH values the nitrogen atoms in these structures (and in subsequent figures) would be predominantly protonated (see Chapter 32). Additional polyamines found in thermophiles are described in Chapter 5.
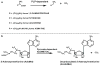
Fig 2
Decarboxylases involved in polyamine synthesis. (a) Pyridoxal phosphate (PLP)-dependent decarboxylases [L-1,4-diaminobutyric acid decarboxylase, L-ornithine decarboxylase (ODC), L-lysine decarboxylase and L-arginine decarboxylase]. (b) _S_-adenosylmethionine decarboxylase, which contains a covalently bound pyruvate group essential for activity.
Fig 3
Aminopropyltransferases involved in polyamine synthesis. The reaction catalyzed by these enzymes involves the attack by the unprotonated terminal N of the amine substrate on the methylene C atom adjacent to the sulfonium center of the dcAdoMet aminopropyl donor. The deprotonation of the attacking N, which is facilitated by residues in the enzyme, and the positive charge on the sulfonium S of dcAdoMet allow the reaction to proceed forming the polyamine product. The reactions shown have all been demonstrated by purified aminopropyltransferases. Other members of this family of enzymes may occur to bring about the synthesis of longer chain polyamines.
Fig 4
Condensation reactions forming polyamines from L-aspartic-β-semialdehyde. Reaction of L-aspartic–β-semialdehyde with putrescine or 1,3-diaminopropane by carboxynorspermidine synthase forms carboxyspermidine or carboxynorspermidine, which are decarboxylated by carboxynorspermidine decarboxylase to form spermidine or _sym_-norspermidine, respectively.
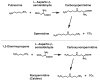
Fig 5
Synthesis of _sym_-homospermine and of hypusine. _sym_-Homospermine can be formed from two molecules of putrescine or from putrescine and spermidine as shown in (a). In both cases, NAD is needed for hydrogen extraction, but is regenerated in the second half of the reaction. The reaction using spermidine and putrescine, which also generates 1,3-diaminopropane, is similar to that used for the hypusine modification of eIF5A shown in (b). In this case, a lysine residue in eIF5A is used instead of putrescine forming deoxyhypusine in eIF5A and free 1,3-diaminopropane. A second enzyme hydroxylates the deoxyhypusine to form the complete hypusine modification (see Chapters 12 and 13 for more details of the reactions forming hypusine).
Fig 6
Reactions catalyzed by SSAT.
Fig 7
Reactions catalyzed by APAO and SMO. APAO has a strong preference for _N_1-acetylated polyamines formed by SSAT releasing _N_-acetyl-3-aminopropanal and SMO is specific for spermine releasing 3-aminopropanal. Both reactions also generate hydrogen peroxide.
Similar articles
- Polyamine metabolism and cancer: treatments, challenges and opportunities.
Casero RA Jr, Murray Stewart T, Pegg AE. Casero RA Jr, et al. Nat Rev Cancer. 2018 Nov;18(11):681-695. doi: 10.1038/s41568-018-0050-3. Nat Rev Cancer. 2018. PMID: 30181570 Free PMC article. Review. - A perspective of polyamine metabolism.
Wallace HM, Fraser AV, Hughes A. Wallace HM, et al. Biochem J. 2003 Nov 15;376(Pt 1):1-14. doi: 10.1042/BJ20031327. Biochem J. 2003. PMID: 13678416 Free PMC article. Review. - Development of polyamine analogs as cancer therapeutic agents.
Thomas T, Balabhadrapathruni S, Gallo MA, Thomas TJ. Thomas T, et al. Oncol Res. 2002;13(3):123-35. Oncol Res. 2002. PMID: 12555742 Review. - Clickable Polyamine Derivatives as Chemical Probes for the Polyamine Transport System.
Vanhoutte R, Kahler JP, Martin S, van Veen S, Verhelst SHL. Vanhoutte R, et al. Chembiochem. 2018 May 4;19(9):907-911. doi: 10.1002/cbic.201800043. Epub 2018 Apr 14. Chembiochem. 2018. PMID: 29451723 - Animal disease models generated by genetic engineering of polyamine metabolism.
Jänne J, Alhonen L, Keinänen TA, Pietilä M, Uimari A, Pirinen E, Hyvönen MT, Järvinen A. Jänne J, et al. J Cell Mol Med. 2005 Oct-Dec;9(4):865-82. doi: 10.1111/j.1582-4934.2005.tb00385.x. J Cell Mol Med. 2005. PMID: 16364196 Free PMC article. Review.
Cited by
- Integrated Multi-Omic Characterization of the Detachment Process of Adherent Vero Cells with Animal-Based and Animal-Origin-Free Enzymes.
Yu S, Huang Y, Wu C, Fu W, Liang H, Chen C, Cheng Y, Guo Y, Zhang Y, Wang H, Yang X. Yu S, et al. Cells. 2022 Oct 27;11(21):3396. doi: 10.3390/cells11213396. Cells. 2022. PMID: 36359792 Free PMC article. - Polyamine metabolism and cancer: treatments, challenges and opportunities.
Casero RA Jr, Murray Stewart T, Pegg AE. Casero RA Jr, et al. Nat Rev Cancer. 2018 Nov;18(11):681-695. doi: 10.1038/s41568-018-0050-3. Nat Rev Cancer. 2018. PMID: 30181570 Free PMC article. Review. - Genome-Wide Identification and Functional Analysis of Polyamine Oxidase Genes in Maize Reveal Essential Roles in Abiotic Stress Tolerance.
Xi Y, Hu W, Zhou Y, Liu X, Qian Y. Xi Y, et al. Front Plant Sci. 2022 Aug 4;13:950064. doi: 10.3389/fpls.2022.950064. eCollection 2022. Front Plant Sci. 2022. PMID: 35991458 Free PMC article. - Deep Resequencing of the 1q22 Locus in Non-Lobar Intracerebral Hemorrhage.
Parodi L, Comeau ME, Georgakis MK, Mayerhofer E, Chung J, Falcone GJ, Malik R, Demel SL, Worrall BB, Koch S, Testai FD, Kittner SJ, McCauley JL, Hall CE, Mayson DJ, Elkind MSV, James ML, Woo D, Rosand J, Langefeld CD, Anderson CD. Parodi L, et al. Ann Neurol. 2024 Feb;95(2):325-337. doi: 10.1002/ana.26814. Epub 2023 Oct 14. Ann Neurol. 2024. PMID: 37787451 Free PMC article. - Polyamine regulation of ion channel assembly and implications for nicotinic acetylcholine receptor pharmacology.
Dhara M, Matta JA, Lei M, Knowland D, Yu H, Gu S, Bredt DS. Dhara M, et al. Nat Commun. 2020 Jun 3;11(1):2799. doi: 10.1038/s41467-020-16629-3. Nat Commun. 2020. PMID: 32493979 Free PMC article.
References
- Cohen SS. A guide to the polyamines. Oxford University Press; New York: 1998.
- Naka Y, Watanabe K, Sagor GH, Niitsu M, Pillai MA, Kusano T, Takahashi Y. Quantitative analysis of plant polyamines including thermospermine during growth and salinity stress. Plant Physiol Biochem. 2010;48:527–533. - PubMed
- Williams-Ashman HG. NICOLAS LOUIS VAUQUELIN (1763-1829) Invest Urol. 1965;2:605–613. - PubMed
- Lee J, Michael AJ, Martynowski D, Goldsmith EJ, Phillips MA. Phylogenetic diversity and the structural basis of substrate specificity in the beta/alpha-barrel fold basic amino acid decarboxylases. J Biol Chem. 2007;282:27115–27125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM-26290/GM/NIGMS NIH HHS/United States
- P30 CA006973/CA/NCI NIH HHS/United States
- CA-51085/CA/NCI NIH HHS/United States
- R01 GM026290/GM/NIGMS NIH HHS/United States
- R01 CA018138/CA/NCI NIH HHS/United States
- CA-98454/CA/NCI NIH HHS/United States
- R37 CA018138/CA/NCI NIH HHS/United States
- CA-018138/CA/NCI NIH HHS/United States
- R01 CA051085/CA/NCI NIH HHS/United States
- R01 CA098454/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources