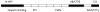Targets for AD treatment: conflicting messages from γ-secretase inhibitors - PubMed (original) (raw)
Targets for AD treatment: conflicting messages from γ-secretase inhibitors
Kumar Sambamurti et al. J Neurochem. 2011 May.
Abstract
Current evidence suggests that Alzheimer's disease (AD) is a multi-factorial disease that starts with accumulation of multiple proteins. We have previously proposed that inhibition of γ-secretase may impair membrane recycling causing neurodegeneration starting at synapses (Sambamurti K., Suram A., Venugopal C., Prakasam A., Zhou Y., Lahiri D. K. and Greig N. H. A partial failure of membrane protein turnover may cause Alzheimer's disease: a new hypothesis. Curr. Alzheimer Res., 3, 2006, 81). We also proposed familal AD mutations increase Aβ42 by inhibiting γ-secretase. Herein, we discuss the failure of Eli Lilly's γ-secretase inhibitor, semagacestat, in clinical trials in the light of our hypothesis, which extends the problem beyond toxicity of Aβ aggregates. We elaborate that γ-secretase inhibitors lead to accumulation of amyloid precursor protein C-terminal fragments that can later be processed by γ-secretase to yields bursts of Aβ to facilitate aggregation. Although we do not exclude a role for toxic Aβ aggregates, inhibition of γ-secretase can affect numerous substrates other than amyloid precursor protein to affect multiple pathways and the combined accumulation of multiple peptides in the membrane may impair its function and turnover. Taken together, protein processing and turnover pathways play an important role in maintaining cellular homeostasis and unless we clearly see consistent disease-related increase in their levels or activity, we need to focus on preserving their function rather than inhibiting them for treatment of AD and similar diseases.
2011 International Society for Neurochemistry. Published 2011. This article is a US Government work and is in the public domain in the USA.
Conflict of interest statement
This research does not represent a conflict of interest for the authors.
Figures
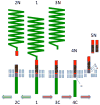
Figure 1. Pathways of APP proteolysis
The full-length APP is a type-I integral membrane protein. It is cleaved in the exocytoplasmic domain at the start of Aβ by β-secretase and between residues 16-17 of Aβ by α secretase to generate secreted derivatives sAPPβ and sAPPα and C-terminal fragments CTFβ and CTFα. γ-Secretase cleaves CTFβ at the membrane cytoplasm boundary (ε site) to CTFγ and Aβ49, and subsequently to Aβ40 and Aβ42. Most secreted Aβ ends at residue 40 but a small percentage is longer terminating at residues 42 and 43. CTFα is similarly processed by γ-secretase (not shown). The predicted CTFγ is not seen probably due to its rapid turnover in the cell.
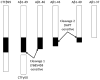
Figure 2. Model for γ-secretase processing of APP
The final step of intramembrane processing is mediated by γ-secretase, which is a multisubunit integral membrane protein. Cleavage of membrane protein is enigmatic due to the lack of water within the membrane for hydrolysis. The proposed model takes advantage of the knowledge that γ-secretase is a multicatalytic enzyme that cleaves CTFα/β multiple times within the membrane and these cleavages are differentially sensitive to two different inhibitors: LY-685,458 and DAPT. The hypothesis suggests that after cleaving at the membrane cytoplasmic boundary, the transmembrane domain is still bound to the first LY-685,458 – sensitive active site of the enzyme and is subsequently free floating within the membrane. At this stage, it is susceptible to cleavage at the membrane - medium interface where it is cleaved by the second DAPT-sensitive active site. Thus, LY-685,458 blocks all cleavages whereas DAPT only blocks the cleavage from Aβ46 to Aβ43 and beyond. Each of these cuts are slightly ragged, but normally follow a pattern of cleaving three residues from the first cleavage.
Figure 3. Multiple toxic domains and metabolites of APP have been defined
Multiple cleavage sites have been defined on APP and these appear to generate a number of toxic proteins such as n-APP, Aβ and C31 shown above the APP molecule in the schematic diagram (not to scale). Even the major cleavage products such as CTFγ/AICD have been defined as toxic in animal models. These potentially toxic fragments demonstrate that the toxicity of APP may arise due to improper turnover and cleavage rather than due to the normal turnover pathways and argue against disrupting normal turnover mechanisms. APP is an important multifunctional protein with the key known functional domains shown below the molecule.
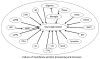
Figure 4. Faulty protein turnover can lead to toxic accumulation of a number of proteins
A number of proteins are known to accumulate in senile plaques and neurofibrillary tangles in subpopulations of patients with AD. Several of these proteins are known to accumulate in other degenerative diseases and have mutations linked to the disease. Some of the accumulated proteins become toxic after microglial processing whereas others are degraded to inactive products by microglia as shown for plaque amyloid. None of these proteins are neurotoxic in the young or even most of the health aging populations. Thus, rather than focusing on toxic products one may need to find ways to preserve the proteolytic clearance pathways that maintain secreted, membrane and cytoplasmic protein homeostasis.
Similar articles
- Interacting with γ-secretase for treating Alzheimer's disease: from inhibition to modulation.
Panza F, Frisardi V, Solfrizzi V, Imbimbo BP, Logroscino G, Santamato A, Greco A, Seripa D, Pilotto A. Panza F, et al. Curr Med Chem. 2011;18(35):5430-47. doi: 10.2174/092986711798194351. Curr Med Chem. 2011. PMID: 22087836 Review. - γ-secretase inhibitors and modulators for the treatment of Alzheimer's disease: disappointments and hopes.
Imbimbo BP, Giardina GA. Imbimbo BP, et al. Curr Top Med Chem. 2011;11(12):1555-70. doi: 10.2174/156802611795860942. Curr Top Med Chem. 2011. PMID: 21510832 Review. - Advances in the identification of γ-secretase inhibitors for the treatment of Alzheimer's disease.
D'Onofrio G, Panza F, Frisardi V, Solfrizzi V, Imbimbo BP, Paroni G, Cascavilla L, Seripa D, Pilotto A. D'Onofrio G, et al. Expert Opin Drug Discov. 2012 Jan;7(1):19-37. doi: 10.1517/17460441.2012.645534. Epub 2011 Dec 14. Expert Opin Drug Discov. 2012. PMID: 22468891 Review. - REVIEW: γ-Secretase inhibitors for the treatment of Alzheimer's disease: The current state.
Panza F, Frisardi V, Imbimbo BP, Capurso C, Logroscino G, Sancarlo D, Seripa D, Vendemiale G, Pilotto A, Solfrizzi V. Panza F, et al. CNS Neurosci Ther. 2010 Oct;16(5):272-84. doi: 10.1111/j.1755-5949.2010.00164.x. Epub 2010 Jun 16. CNS Neurosci Ther. 2010. PMID: 20560993 Free PMC article. Review. - Therapeutic intervention for Alzheimer's disease with γ-secretase inhibitors: still a viable option?
Imbimbo BP, Panza F, Frisardi V, Solfrizzi V, D'Onofrio G, Logroscino G, Seripa D, Pilotto A. Imbimbo BP, et al. Expert Opin Investig Drugs. 2011 Mar;20(3):325-41. doi: 10.1517/13543784.2011.550572. Epub 2011 Jan 11. Expert Opin Investig Drugs. 2011. PMID: 21222550 Review.
Cited by
- Neuroprotective Strategies and Cell-Based Biomarkers for Manganese-Induced Toxicity in Human Neuroblastoma (SH-SY5Y) Cells.
Cahill CM, Sarang SS, Bakshi R, Xia N, Lahiri DK, Rogers JT. Cahill CM, et al. Biomolecules. 2024 May 31;14(6):647. doi: 10.3390/biom14060647. Biomolecules. 2024. PMID: 38927051 Free PMC article. - Translational inhibition of APP by Posiphen: Efficacy, pharmacodynamics, and pharmacokinetics in the APP/PS1 mouse.
Teich AF, Sharma E, Barnwell E, Zhang H, Staniszewski A, Utsuki T, Padmaraju V, Mazell C, Tzekou A, Sambamurti K, Arancio O, Maccecchini ML. Teich AF, et al. Alzheimers Dement (N Y). 2018 Jan 18;4:37-45. doi: 10.1016/j.trci.2017.12.001. eCollection 2018. Alzheimers Dement (N Y). 2018. PMID: 29955650 Free PMC article. - Targeting protein aggregation for the treatment of degenerative diseases.
Eisele YS, Monteiro C, Fearns C, Encalada SE, Wiseman RL, Powers ET, Kelly JW. Eisele YS, et al. Nat Rev Drug Discov. 2015 Nov;14(11):759-80. doi: 10.1038/nrd4593. Epub 2015 Sep 4. Nat Rev Drug Discov. 2015. PMID: 26338154 Free PMC article. Review. - Gelsolin amyloidosis: genetics, biochemistry, pathology and possible strategies for therapeutic intervention.
Solomon JP, Page LJ, Balch WE, Kelly JW. Solomon JP, et al. Crit Rev Biochem Mol Biol. 2012 May-Jun;47(3):282-96. doi: 10.3109/10409238.2012.661401. Epub 2012 Feb 24. Crit Rev Biochem Mol Biol. 2012. PMID: 22360545 Free PMC article. Review.
References
- Anderson JP, Esch FS, Keim PS, Sambamurti K, Lieberburg I, Robakis NK. Exact cleavage site of Alzheimer amyloid precursor in neuronal PC-12 cells. Neurosci Lett. 1991;128:126–128. - PubMed
- Bancher C, Lassmann H, Budka H, Grundke-Iqbal I, Iqbal K, Wiche G, Seitelberger F, Wisniewski HM. Neurofibrillary tangles in Alzheimer’s disease and progressive supranuclear palsy: antigenic similarities and differences. Microtubule-associated protein tau antigenicity is prominent in all types of tangles. Acta Neuropathol. 1987;74:39–46. - PubMed
- Baudier J, Cole RD. Phosphorylation of tau proteins to a state like that in Alzheimer’s brain is catalyzed by a calcium/calmodulin-dependent kinase and modulated by phospholipids. J Biol Chem. 1987;262:17577–17583. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG018884/AG/NIA NIH HHS/United States
- R01 AG023055/AG/NIA NIH HHS/United States
- R01 AG023055-05/AG/NIA NIH HHS/United States
- AG022103/AG/NIA NIH HHS/United States
- R01 AG022103-05/AG/NIA NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R01 AG018379/AG/NIA NIH HHS/United States
- AG18379/AG/NIA NIH HHS/United States
- AG023055/AG/NIA NIH HHS/United States
- R01 AG018884-09/AG/NIA NIH HHS/United States
- AG18884/AG/NIA NIH HHS/United States
- R01 AG018379-10/AG/NIA NIH HHS/United States
- R01 NS051575/NS/NINDS NIH HHS/United States
- R01 AG022103/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical