Depletion of key protein components of the RISC pathway impairs pre-ribosomal RNA processing - PubMed (original) (raw)
Depletion of key protein components of the RISC pathway impairs pre-ribosomal RNA processing
Xue-Hai Liang et al. Nucleic Acids Res. 2011 Jun.
Abstract
Little is known about whether components of the RNA-induced silencing complex (RISC) mediate the biogenesis of RNAs other than miRNA. Here, we show that depletion of key proteins of the RISC pathway by antisense oligonucleotides significantly impairs pre-rRNA processing in human cells. In cells depleted of Drosha or Dicer, different precursors to 5.8S rRNA strongly accumulated, without affecting normal endonucleolytic cleavages. Moderate yet distinct processing defects were also observed in Ago2-depleted cells. Physical links between pre-rRNA and these proteins were identified by co-immunoprecipitation analyses. Interestingly, simultaneous depletion of Dicer and Drosha led to a different processing defect, causing slower production of 28S rRNA and its precursor. Both Dicer and Ago2 were detected in the nuclear fraction, and reduction of Dicer altered the structure of the nucleolus, where pre-rRNA processing occurs. Together, these results suggest that Drosha and Dicer are implicated in rRNA biogenesis.
Figures
Figure 1.
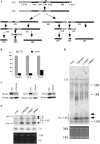
Pre-rRNA accumulation in cells depleted of RISC pathway proteins. (A) Pre-rRNA processing pathway in mammals. ETS and ITS are external and internal transcribed spacers, respectively. The position of the hybridization probe used in (D and E) is shown as a solid bar above 47S pre-rRNA. (B) mRNA levels were dramatically reduced 48 h after treatment with 50 nM ASOs targeting Drosha (ISIS25690), Ago2 (ISIS136764) or Dicer (ISIS138648), as determined by qRT–PCR. The error bars indicate standard deviation from two independent experiments with three replicates. UTC, untreated cells; +ASO, cells treated with ASOs. (C) The levels of targeted proteins were significantly reduced by ASO treatment, as determined by western analysis. Alpha-tubulin was used as a control for loading. (D) Northern hybridization for pre-rRNA species using a probe specific to the boundary of 5.8S rRNA/ITS2. Total RNA prepared from test cells 48 h after ASO treatment was separated on a 1.2% agarose gel, and the blot was subjected to hybridization. The arrows indicate precursors to 5.8S rRNA. The positions of mature rRNAs are indicated. Lower panel shows ethidium bromide staining of rRNAs in the same gel. (E) Pre-5.8S rRNA accumulated in cells depleted of Drosha, Ago2 or Dicer. Total RNA as used in (C) was separated in an 8% polyacrylamide, 7M urea gel and the blot was hybridized using the same probe as in (D). The arrows indicate different pre-5.8S rRNA species (marked as A, B and C). U3 snoRNA was probed to serve as a loading control. Lower panel shows ethidium bromide staining of rRNAs in the same gel.
Figure 2.
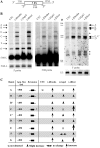
Loss of RISC proteins leads to accumulation of different pre-5.8S rRNA species. (A) Depiction of probe positions around the 5.8S rRNA region. The probe names are shown under the pre-rRNA. (B) Total RNA was prepared from test cells 48 h after ASO treatment, and subjected to northern hybridization. The same membrane was hybridized sequentially using probes specific to 5′ (left panel, 5′ probe), 5.8S (middle panel, 5.8S probe) and 3′ regions (right panel, 3′ probe), respectively. U3 snoRNA was used as a loading control. Different pre-5.8S rRNA species are indicated. The asterisks indicate RNA species that were only detected with one probe. M, size maker in nucleotides (low range ssRNA ladder, Biolabs); 5.8S-L, the longer version of mature 5.8S rRNA; shorter exposure for 5.8S-L rRNA is shown in left panel, lower part. (C) Summary of changes in the levels of different pre-5.8S rRNA species in cells depleted of the RISC pathway proteins. App. size, estimated nucleotide length of the RNA. Extension, types of 5′ or 3′ flanking sequences in pre-5.8S rRNAs. The larger arrows indicate stronger accumulation.
Figure 3.
Depletion of Dicer or Drosha does not block normal endonucleolytic cleavages in pre-rRNA. (A) Relative positions of primers used in primer extension assays. The numbered arrows below the pre-rRNA represent the primers. Positions relative to 5.8S rRNA are shown. (B) Primer extension analysis for ITS1 region using primer 2. The extension products were separated in an 8% sequence gel, next to a primer extension sequencing ladder performed with a primer specific to 18S rRNA (XL066). The same sequencing ladder was used for all gels in this figure. The calculated distances relative to the 5′-end of 5.8S rRNA are given in nucleotides. The solid arrows indicate normal processing products, whereas open arrows indicate products accumulated only in Dicer- or Drosha-depleted cells. (C) Primer extension for ITS2 regions using primers 5 and 6, as in (B). The two potential cleavage products are marked by arrows and the distances relative to the 3′-end of 5.8S rRNA are shown in nucleotides. (D and E) Primer extension using primers 3 and 4, respectively. The arrows indicate pre-rRNA species with 5′-ends that exist in normal cells, but accumulated in Dicer- or Drosha-depleted cells. The asterisks indicate extension stops that occurred within the 5.8S rRNA coding regions. (F) Summary of the mapping data. The solid bars below the pre-rRNA represent positions of primers, as indicated in (A). The potential cleavage positions in normal cells are marked with downward arrows and the relative distances to 5.8S rRNA are shown. The open arrows indicate 5′ positions of pre-rRNAs that accumulated in Dicer-depleted cells, whereas the upward sharp arrows indicate 5′ positions of pre-rRNAs that accumulated in Drosha-depleted cells.
Figure 4.
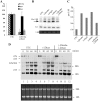
Simultaneous depletion of Drosha and Dicer leads to processing defects different from loss of a single protein. (A) mRNA levels in control cells or in cells depleted of Drosha and Dicer were determined using qRT–PCR, as described in Figure 1. The error bars indicate standard deviation from three independent experiments. (B) Northern hybridization for pre-5.8S rRNA, as in Figure 1. The arrow indicates the pre-5.8S rRNA product used for quantification in panel C. 5.8S rRNA was detected by ethidium bromide staining. U6 snRNA was used as a loading control. (C) The levels of pre-5.8S rRNA detected in (B) was normalized to U6 snRNA and plotted. (D) Double depletion of Dicer and Drosha reduced the processing rates for 32S pre-rRNA and 28S rRNA. In vivo pulse-chase labeling was performed as described in ‘Materials and Methods’ section. Various rRNA species are indicated. The chase times (in minutes) are given above each lane. The lower panel shows ethidium bromide staining of rRNAs in the same gel.
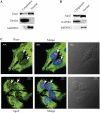
Figure 5.
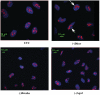
Cells lacking Dicer exhibit altered nucleolar structure. Indirect immunofluorescence was performed for cells depleted of Dicer, Drosha or Ago2 using first antibody against nucleolin and secondary anti-rabbit antibody conjugated to Texas Red (red). DNA was stained with DAPI (blue). Arrows indicate aggregated nucleoli in cells depleted of Dicer.
Figure 6.
Pre-rRNA containing 5.8S rRNA and flanking ITS sequences can be co-immunoprecipitated with RISC proteins. Immunoprecipitation was carried out using antibodies against Drosha, Ago2 or Dicer. Co-selected RNAs were analyzed by RT–PCR. (A) The positions of probe sets in pre-rRNA used for RT–PCR reaction are indicated. The expected sizes of PCR product are given. Co-immunoprecipitated RNAs were subjected to reverse transcription with (+RT) or without (–RT) oligonucleotides complementary to different regions of pre-rRNA or tubulin mRNA. RT reactions were used as templates for PCR amplification. The PCR products were analyzed on 2% agarose gels. Input, RNA prepared from 10% of the material used for immunoprecipitation. –AB, control immunoprecipitation experiment without antibody; M, 1 kb plus DNA ladder. The sizes are shown in base pairs. (B and C) RT–PCR detection for pre-rRNAs containing 5.8S/ITS2 and ITS1/5.8S regions, respectively. (D) RT–PCR for 18S/ITS1 region (upper panel) or 28S/3′ETS region (lower panel). (E) RT–PCR for β-tubulin mRNA. (F) Immunoprecipitation was performed using an antibody against a splicing factor (SF3B3), and the precipitated RNA was subjected to RT–PCR for ITS1/5.8S (3′ITS1) or 5.8S/ITS2 (ITS2) regions.
Figure 7.
Nuclear localization of Dicer and Ago2. (A) Western analysis of Dicer in cytoplasmic and nuclear fractions. The 5% cytoplasmic and 20% nuclear fractions prepared from HeLa cells were loaded in a 4–12% SDS–PAGE gel, transferred to a membrane and proteins were detected using antibodies. Alpha-tubulin and hnRNP A2 were used as controls for cytoplasmic and nuclear proteins, respectively. (B) Ago2 can be found in both cytoplasmic and nuclear fractions. Western analysis was performed using the same samples as in (C), and the membrane was probed using different antibodies. GAPDH and hnRNPA2 were detected and used as controls for cytoplasmic and nuclear proteins, respectively. (C) Localization of Dicer (upper panel) and Ago2 (lower panel) in Hela cells. Cells grown on glass-bottom dishes were fixed, stained with first antibodies against Dicer (1:200, ab14601, from mouse, Abcam) and Nucleolin (1:200, ab22758, from rabbit, Abcam), or against Ago2 (1:150, ab57113, from mouse, Abcam) and Nucleolin, as described in ‘Materials and Methods’ section. Secondary antibodies were anti-mouse antibody (1:200, ab6785, Abcam, conjugated with FITC (green) and anti-rabbit antibody (1:200, Ab6719, conjugated with Texas Red). Nucleolin (red) was detected and served as a nucleolar marker. The nucleus was stained with DAPI (blue). The arrow indicates the positions of nucleoli. The scale bars: 10 µm.
Similar articles
- A human, ATP-independent, RISC assembly machine fueled by pre-miRNA.
Maniataki E, Mourelatos Z. Maniataki E, et al. Genes Dev. 2005 Dec 15;19(24):2979-90. doi: 10.1101/gad.1384005. Genes Dev. 2005. PMID: 16357216 Free PMC article. - Expanded RNA-binding activities of mammalian Argonaute 2.
Tan GS, Garchow BG, Liu X, Yeung J, Morris JP 4th, Cuellar TL, McManus MT, Kiriakidou M. Tan GS, et al. Nucleic Acids Res. 2009 Dec;37(22):7533-45. doi: 10.1093/nar/gkp812. Nucleic Acids Res. 2009. PMID: 19808937 Free PMC article. - Bop1 is a mouse WD40 repeat nucleolar protein involved in 28S and 5. 8S RRNA processing and 60S ribosome biogenesis.
Strezoska Z, Pestov DG, Lau LF. Strezoska Z, et al. Mol Cell Biol. 2000 Aug;20(15):5516-28. doi: 10.1128/MCB.20.15.5516-5528.2000. Mol Cell Biol. 2000. PMID: 10891491 Free PMC article. - Post-transcriptional control of miRNA biogenesis.
Michlewski G, Cáceres JF. Michlewski G, et al. RNA. 2019 Jan;25(1):1-16. doi: 10.1261/rna.068692.118. Epub 2018 Oct 17. RNA. 2019. PMID: 30333195 Free PMC article. Review. - The multifunctional nucleolus.
Boisvert FM, van Koningsbruggen S, Navascués J, Lamond AI. Boisvert FM, et al. Nat Rev Mol Cell Biol. 2007 Jul;8(7):574-85. doi: 10.1038/nrm2184. Nat Rev Mol Cell Biol. 2007. PMID: 17519961 Review.
Cited by
- Discovery of novel microRNA mimic repressors of ribosome biogenesis.
Bryant CJ, McCool MA, Rosado González GT, Abriola L, Surovtseva YV, Baserga SJ. Bryant CJ, et al. Nucleic Acids Res. 2024 Feb 28;52(4):1988-2011. doi: 10.1093/nar/gkad1235. Nucleic Acids Res. 2024. PMID: 38197221 Free PMC article. - RNase III, Ribosome Biogenesis and Beyond.
Lejars M, Kobayashi A, Hajnsdorf E. Lejars M, et al. Microorganisms. 2021 Dec 17;9(12):2608. doi: 10.3390/microorganisms9122608. Microorganisms. 2021. PMID: 34946208 Free PMC article. Review. - Genetic Insight into the Domain Structure and Functions of Dicer-Type Ribonucleases.
Ciechanowska K, Pokornowska M, Kurzyńska-Kokorniak A. Ciechanowska K, et al. Int J Mol Sci. 2021 Jan 9;22(2):616. doi: 10.3390/ijms22020616. Int J Mol Sci. 2021. PMID: 33435485 Free PMC article. Review. - A Tetrahymena Piwi bound to mature tRNA 3' fragments activates the exonuclease Xrn2 for RNA processing in the nucleus.
Couvillion MT, Bounova G, Purdom E, Speed TP, Collins K. Couvillion MT, et al. Mol Cell. 2012 Nov 30;48(4):509-20. doi: 10.1016/j.molcel.2012.09.010. Epub 2012 Oct 18. Mol Cell. 2012. PMID: 23084833 Free PMC article. - Identification and characterization of intracellular proteins that bind oligonucleotides with phosphorothioate linkages.
Liang XH, Sun H, Shen W, Crooke ST. Liang XH, et al. Nucleic Acids Res. 2015 Mar 11;43(5):2927-45. doi: 10.1093/nar/gkv143. Epub 2015 Feb 20. Nucleic Acids Res. 2015. PMID: 25712094 Free PMC article.
References
- Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS. Post-transcriptional gene silencing by siRNAs and miRNAs. Curr. Opin. Struct. Biol. 2005;15:331–341. - PubMed
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell. Bio.l. 2009;10:126–139. - PubMed
- Mallory AC, Bouche N. MicroRNA-directed regulation: to cleave or not to cleave. Trends Plant Sci. 2008;13:359–367. - PubMed
- Seitz H, Zamore PD. Rethinking the microprocessor. Cell. 2006;125:827–829. - PubMed
- Chekulaeva M, Filipowicz W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr. Opin. Cell Biol. 2009;21:452–460. - PubMed