Polycystin-2 takes different routes to the somatic and ciliary plasma membrane - PubMed (original) (raw)
Polycystin-2 takes different routes to the somatic and ciliary plasma membrane
Helen Hoffmeister et al. J Cell Biol. 2011.
Abstract
Polycystin-2 (also called TRPP2), an integral membrane protein mutated in patients with cystic kidney disease, is located in the primary cilium where it is thought to transmit mechanical stimuli into the cell interior. After studying a series of polycystin-2 deletion mutants we identified two amino acids in loop 4 that were essential for the trafficking of polycystin-2 to the somatic (nonciliary) plasma membrane. However, polycystin-2 mutant proteins in which these two residues were replaced by alanine were still sorted into the cilium, thus indicating that the trafficking routes to the somatic and ciliary plasma membrane compartments are distinct. We also observed that the introduction of dominant-negative Sar1 mutant proteins and treatment of cells with brefeldin A prevented the transport into the ciliary plasma membrane compartment, whereas metabolic labeling experiments, light microscopical imaging, and high-resolution electron microscopy revealed that full-length polycystin-2 did not traverse the Golgi apparatus on its way to the cilium. These data argue that the transport of polycystin-2 to the ciliary and to the somatic plasma membrane compartments originates in a COPII-dependent fashion at the endoplasmic reticulum, that polycystin-2 reaches the cis side of the Golgi apparatus in either case, but that the trafficking to the somatic plasma membrane goes through the Golgi apparatus whereas transport vesicles to the cilium leave the Golgi apparatus at the cis compartment. Such an interpretation is supported by the finding that mycophenolic acid treatment resulted in the colocalization of polycystin-2 with GM130, a marker of the cis-Golgi apparatus. Remarkably, we also observed that wild-type Smoothened, an integral membrane protein involved in hedgehog signaling that under resting conditions resides in the somatic plasma membrane, passed through the Golgi apparatus, but the M2 mutant of Smoothened, which is constitutively located in the ciliary but not in the somatic plasma membrane, does not. Finally, a dominant-negative form of Rab8a, a BBSome-associated monomeric GTPase, prevented the delivery of polycystin-2 to the primary cilium whereas a dominant-negative form of Rab23 showed no inhibitory effect, which is consistent with the view that the ciliary trafficking of polycystin-2 is regulated by the BBSome.
Figures
Figure 1.
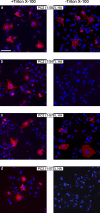
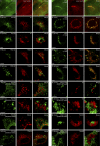
Evidence for an export motif in polycystin-2. COS-7 cells were transiently transfected with expression plasmids for the polycystin-2 mutant proteins indicated. 2 d later the cells were stained with an antibody directed against an extracellular domain (loop 1) of polycystin-2 (red) in the presence and absence of the detergent Triton X-100. Only when the mutant proteins reached the plasma membrane could a signal be detected in the absence of Triton X-100. Nuclei were counterstained with Hoechst 33258 (blue) to demonstrate the presence of cells. Bar, 100 µm.
Figure 2.
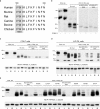
Endoglycosidase H sensitivity and cell surface biotinylation of mutated polycystin-2 proteins. (a) Sequence comparison of polycystin-2 from various species. Shown are amino acids 569–580 of the human protein; the last three amino acids of the presumptive fourth membrane-spanning segment (TMD4) are highlighted in gray. (b) Endoglycosidase Hf (Endo H) assay of HA epitope–tagged full-length polycystin-2 (PC2, HA) and of polycystin-2 (1–703) without additional mutation (No mut.) and with alanine substitutions at positions 572–574 and 575–577. The full-length polycystin-2 protein and the triple-alanine substitution mutants are fully sensitive to treatment with Endo H, but the polycystin-2 (1–703) protein without additional mutations is not (asterisk indicates the resistant band). The band indicated by the arrow probably represents full-length polycystin-2 that did not enter the gel. (c–e) Surface biotinylation of COS-7 (c and e) and LLC-PK1 cells (d) transiently transfected with expression plasmids for the indicated proteins. The α1 subunit of the Na+/K+-ATPase served as a control for efficient biotinylation of plasma membrane proteins. When proteins were (mostly) retained in intracellular membrane compartments, no signal or only a weak signal was detected after precipitation with NeutrAvidin beads. The arrow in d indicates a nonspecific band. Molecular masses (in kD) are indicated on the left of panels b–e. L, whole-cell lysate; B, proteins precipitated with NeutrAvidin beads; S, proteins left in the supernatant after precipitation with NeutrAvidin beads.
Figure 3.
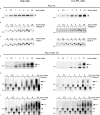
Pulse-chase labeling of full-length polycystin-2 and polycystin-2 (1–703). Stably transfected HeLa and LLC-PK1 cells inducibly producing HA epitope–tagged full-length polycystin-2 (a–c) and polycystin-2 (1–703; d–f) proteins were metabolically labeled with 35S-methionine/cysteine. The HA-tagged proteins were immunoprecipitated with an anti-HA epitope antibody, digested with PNGase F and endoglycosidase H (Endo H) when indicated, run on a polyacrylamide gel, and visualized by autoradiography. (a–c) The full-length polycystin-2 can be digested with both glycosidases at any time point after the pulse. Because the top band (filled arrowhead) disappeared after a digest with calf intestinal phosphatase (not depicted), it represents a phosphorylated form of polycystin-2. (d–f) The truncated polycystin-2 protein migrated as a smear (large arrow) ∼2 h after the pulse. The smear was resistant to endoglycosidase H and therefore probably represents protein that has progressed beyond the cis-Golgi apparatus. Because both bottom bands (filled and open arrowhead) collapsed into one faster-migrating band (small arrow) after endoglycosidase H treatment, they represent a pool of truncated polycystin-2 that did not reach the mid-Golgi apparatus. Molecular masses (in kD) are indicated on the left of each panel.
Figure 4.
Ciliary trafficking of polycystin-2 mutant proteins. LLC-PK1 cells were transiently transfected with expression plasmids for HA epitope–tagged polycystin-2 (1–703) proteins without and with the indicated alanine substitutions. 3–4 d after transfection, cells were double stained for the HA epitope and for the primary cilia marker acetylated tubulin. Primary cilia are indicated by arrows. Bar, 15 µm.
Figure 5.
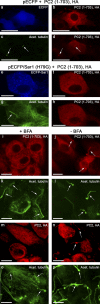
The ciliary trafficking of polycystin-2 requires COPII and an intact Golgi apparatus. (a–h) LLC-PK1 cells were transiently transfected with 8 µg of an expression plasmid for ECFP (a–d) or a fusion protein between ECFP and the dominant-negative Sar1 (H79G) protein (e–h) and with 6 µg of an expression plasmid for HA epitope–tagged polycystin-2 (1–703). 3 d after transfection, the cells were either stained for the HA epitope or double-stained for the HA epitope and acetylated tubulin. Whereas ECFP had no effect on the ciliary location of the truncated polycystin-2 protein, the ECFP-Sar1 (H79G) fusion protein prevented the trafficking of the truncated polycystin-2 into primary cilia. a/b, c/d, e/f, and g/h represent corresponding panels. (i–p) LtA-2,22 cells were stably transfected with expression plasmids for HA epitope–tagged full-length and polycystin-2 (1–703) proteins, and treated with brefeldin A (+BFA; i, k, m, and o) or with the solvent methanol (−BFA; j, l, n, and p). Double staining for the HA epitope and acetylated tubulin revealed that in the presence of brefeldin A the truncated and the full-length polycystin-2 protein no longer reached primary cilia. i/k, j/l, m/o, and n/p represent corresponding panels. Primary cilia are indicated by arrows. Bars: (a–h) 25 µm, (i–p) 10 µm.
Figure 6.
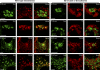
Trafficking of full-length polycystin-2 and polycystin-2 (1–703) with respect to the Golgi apparatus and the trans-Golgi network. (a and j) Immunostaining for acetylated tubulin (red) demonstrates that EGFP fusion proteins with full-length polycystin-2 (PC (fl)) and truncated polycystin-2 (PC2 (1–703)) still enter the primary cilium. (b–e and k–n) LLC-PK1 cells stably synthesizing ECFP fusion proteins with _N_-acetylgalactosaminyltransferase-2 (T2) and TGN38 (shown in red for better visibility) were transiently transfected with expression plasmids coding for an EGFP fusion protein with the respective polycystin-2 proteins. 3–4 d later, the cells were incubated for 5 h at 15°C before being incubated at 37°C. Immediately before (0 min) and 40 min after (40 min) shifting the cells from 15°C to 37°C, the cells were fixed and visualized in a confocal laser scanning microscope. No overlapping signal can be seen between full-length polycystin-2 and the two marker proteins (b–e), whereas a good overlap was observed for the truncated polycystin-2 protein (k–n). (f–I and o–r) Analogous experiments were performed for the (KFI575-577AAA) mutants of full-length polycystin-2 and polycystin-2 (1–703). For neither mutant protein were we able to detect a colocalization with _N_-acetylgalactosaminyltransferase-2 and TGN38. Bar, 5 µm.
Figure 7.
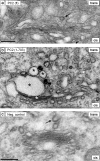
Ultrastructural localization of full-length polycystin-2 and polycystin-2 (1–703). COS-7 cells were transiently transfected with expression plasmids for full-length polycystin-2 (a) and polycystin-2 (1–703) (b and c) fused with EGFP and the tetracysteine motif. (a and b) Cells with EGFP fluorescence. Asterisks in b indicate Golgi cisternae with an osmiophilic diaminobenzidine precipitate in a cell producing the truncated polycystin-2 protein. No such precipitate can be seen in a cell producing full-length polycystin-2. (c) A cell without EGFP fluorescence served to demonstrate background staining. The arrows in a and c point to clathrin-coated budding vesicles marking the trans-Golgi network. Bar, 200 nm.
Figure 8.
Trafficking of wild-type Smoothened and the M2 mutant of Smoothened. LLC-PK1 cells stably synthesizing ECFP fusion proteins with _N_-acetylgalactosaminyltransferase-2 (T2) and TGN38 (shown in red for better visibility) were transiently transfected with expression plasmids coding for an EYFP fusion protein with the respective Smoothened proteins (shown in green for better visibility). 3–4 d later, the cells were incubated for 5 h at 15°C before being incubated at 37°C. Immediately before (0 min) as well as 15 min (15 min) and 40 min (40 min) after shifting the cells from 15°C to 37°C, the cells were fixed and visualized in a confocal laser scanning microscope. Overlapping signals were seen between wild-type Smoothened and the two marker proteins (a–d), whereas no overlap was observed for the M2 mutant of Smoothened (e–h). Bar, 5 µm.
Figure 9.
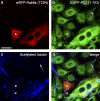
Rab8a is necessary for the trafficking of polycystin-2 into the primary cilium. Stably transfected LLC-PK1 cells inducibly synthesizing a fusion protein between EGFP and polycystin-2 (1–703) were microinjected with an expression plasmid encoding a fusion protein between RFP and the dominant-negative Rab8a T22N mutant protein. The cell marked by an asterisk synthesizes both Rab8a T22N (a) and polycystin-2 (1–703) (b), but its primary cilium (c and d, arrowhead) does not contain polycystin-2 (1–703). In other cells not synthesizing Rab8a T22N, the primary cilium is positive for polycystin-2 (1–703) (b–d, arrow). Bar, 20 µm.
Figure 10.
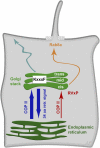
Model for the trafficking of polycystin-2 to the primary cilium. Most of the wild-type polycystin-2 protein leaves the ER in a COPII-dependent fashion and is rapidly returned from the Golgi apparatus due to the presence of a 34–amino acid retrieval signal in its C terminus (dark blue arrows). A small portion of wild-type polycystin-2 will reach the cis-Golgi apparatus (red arrow) and is diverted to the primary cilium (orange arrow), where the delivery is controlled by Rab8a. Whether the ciliary targeting signal “RVxP” in the N terminus of polycystin-2 acts at the cis-Golgi compartment or at a more distal location is not known. Under some circumstances, e.g., due to the interaction between polycystin-2 and polycystin-1 or upon the loss of the retrieval signal in the C terminus, polycystin-2 will also reach the somatic plasma membrane through the Golgi apparatus (light blue arrow). Passage through the Golgi apparatus depends on the “KxxxF” motif in loop 4.
Similar articles
- Regulation of polycystin expression, maturation and trafficking.
Hu J, Harris PC. Hu J, et al. Cell Signal. 2020 Aug;72:109630. doi: 10.1016/j.cellsig.2020.109630. Epub 2020 Apr 8. Cell Signal. 2020. PMID: 32275942 Free PMC article. Review. - Golgi bypass of ciliary proteins.
Witzgall R. Witzgall R. Semin Cell Dev Biol. 2018 Nov;83:51-58. doi: 10.1016/j.semcdb.2018.03.010. Epub 2018 Mar 24. Semin Cell Dev Biol. 2018. PMID: 29559335 Review. - Newly synthesized polycystin-1 takes different trafficking pathways to the apical and ciliary membranes.
Gilder AL, Chapin HC, Padovano V, Hueschen CL, Rajendran V, Caplan MJ. Gilder AL, et al. Traffic. 2018 Dec;19(12):933-945. doi: 10.1111/tra.12612. Epub 2018 Sep 24. Traffic. 2018. PMID: 30125442 Free PMC article. - The heteromeric PC-1/PC-2 polycystin complex is activated by the PC-1 N-terminus.
Ha K, Nobuhara M, Wang Q, Walker RV, Qian F, Schartner C, Cao E, Delling M. Ha K, et al. Elife. 2020 Nov 9;9:e60684. doi: 10.7554/eLife.60684. Elife. 2020. PMID: 33164752 Free PMC article. - A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1.
Ward HH, Brown-Glaberman U, Wang J, Morita Y, Alper SL, Bedrick EJ, Gattone VH 2nd, Deretic D, Wandinger-Ness A. Ward HH, et al. Mol Biol Cell. 2011 Sep;22(18):3289-305. doi: 10.1091/mbc.E11-01-0082. Epub 2011 Jul 20. Mol Biol Cell. 2011. PMID: 21775626 Free PMC article.
Cited by
- The cAMP Signaling Pathway and Direct Protein Kinase A Phosphorylation Regulate Polycystin-2 (TRPP2) Channel Function.
Cantero Mdel R, Velázquez IF, Streets AJ, Ong AC, Cantiello HF. Cantero Mdel R, et al. J Biol Chem. 2015 Sep 25;290(39):23888-96. doi: 10.1074/jbc.M115.661082. Epub 2015 Aug 12. J Biol Chem. 2015. PMID: 26269590 Free PMC article. - TMED2 binding restricts SMO to the ER and Golgi compartments.
Di Minin G, Holzner M, Grison A, Dumeau CE, Chan W, Monfort A, Jerome-Majewska LA, Roelink H, Wutz A. Di Minin G, et al. PLoS Biol. 2022 Mar 30;20(3):e3001596. doi: 10.1371/journal.pbio.3001596. eCollection 2022 Mar. PLoS Biol. 2022. PMID: 35353806 Free PMC article. - Ciliopathies: the trafficking connection.
Madhivanan K, Aguilar RC. Madhivanan K, et al. Traffic. 2014 Oct;15(10):1031-56. doi: 10.1111/tra.12195. Epub 2014 Aug 11. Traffic. 2014. PMID: 25040720 Free PMC article. Review. - A polycystin-centric view of cyst formation and disease: the polycystins revisited.
Ong AC, Harris PC. Ong AC, et al. Kidney Int. 2015 Oct;88(4):699-710. doi: 10.1038/ki.2015.207. Epub 2015 Jul 22. Kidney Int. 2015. PMID: 26200945 Free PMC article. Review. - An intelligent nano-antenna: Primary cilium harnesses TRP channels to decode polymodal stimuli.
Phua SC, Lin YC, Inoue T. Phua SC, et al. Cell Calcium. 2015 Oct;58(4):415-22. doi: 10.1016/j.ceca.2015.03.005. Epub 2015 Mar 21. Cell Calcium. 2015. PMID: 25828566 Free PMC article. Review.
References
- Abramoff M.D., Magelhaes P.J., Ram S.J. 2004. Image processing with ImageJ. Biophotonics International. 11:36–42
- Ausubel F.A., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A., Struhl K. 1996. Current Protocols in Molecular Biology. John Wiley & Sons, NY
- Barr M.M., Sternberg P.W. 1999. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature. 401:386–389 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources