Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine - PubMed (original) (raw)
Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine
Khursheed Iqbal et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Genome-wide erasure of DNA cytosine-5 methylation has been reported to occur along the paternal pronucleus in fertilized oocytes in an apparently replication-independent manner, but the mechanism of this reprogramming process has remained enigmatic. Recently, considerable amounts of 5-hydroxymethylcytosine (5hmC), most likely derived from enzymatic oxidation of 5-methylcytosine (5mC) by TET proteins, have been detected in certain mammalian tissues. 5hmC has been proposed as a potential intermediate in active DNA demethylation. Here, we show that in advanced pronuclear-stage zygotes the paternal pronucleus contains substantial amounts of 5hmC but lacks 5mC. The converse is true for the maternal pronucleus, which retains 5mC but shows little or no 5hmC signal. Importantly, 5hmC persists into mitotic one-cell, two-cell, and later cleavage-stage embryos, suggesting that 5mC oxidation is not followed immediately by genome-wide removal of 5hmC through excision repair pathways or other mechanisms. This conclusion is supported by bisulfite sequencing data, which shows only limited conversion of modified cytosines to cytosines at several gene loci. It is likely that 5mC oxidation is carried out by the Tet3 oxidase. Tet3, but not Tet1 or Tet2, was expressed at high levels in oocytes and zygotes, with rapidly declining levels at the two-cell stage. Our results show that 5mC oxidation is part of the early life cycle of mammals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
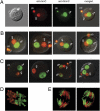
5hmC is present in the male pronucleus of mouse zygotes. (A) A mouse zygote was double-stained with anti-5hmC antibody (green) and anti-5mC antibody (red). The smaller maternal pronucleus is closer to the polar body (pb). A bright-field image is shown on the far left. (B) Additional zygotes were double-stained with anti-5hmC antibody (green) and anti-5mC antibody (red). Merged images are shown. (C) Zygotes obtained by in vitro fertilization were double-stained similarly. Two polyspermic zygotes (to the right) exhibit 5hmC staining in two paternal pronuclei. (D) 5mC and 5hmC staining reveal two separate chromosome sets at metaphase of zygote division. A confocal image is shown. (E) Individual chromosomes are largely stained for either 5mC (likely originated from the maternal pronucleus) or 5hmC (likely from the paternal pronucleus) at anaphase of zygote division. Two Z sections of the same zygote are shown.
Fig. 2.
5hmC and 5mC in early pronuclear stage zygotes. (A) Zygotes at pronuclear stages PN1, PN2, and PN3 were double-stained with anti-5hmC antibody (green) and anti-5mC antibody (red). Merged images are shown. (B) The levels of 5hmC and 5mC in paternal and maternal pronuclei were quantitated. The ratio of staining signal between the paternal and maternal pronucleus is plotted. The number of zgotes analyzed in PN1/PN2 (Early), in PN3 (Mid), and in PN4/PN5 (Late) are indicated with n values. The median value is indicated by a horizontal line and a number. The difference between each two datasets is statistically significant, as seen in the P values of _t_-tests.
Fig. 3.
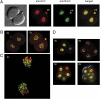
5hmC and 5mC in early cleavage-stage embryos. (A) Two-cell stage embryos were double-stained with anti-5hmC antibody (green) and anti-5mC antibody (red). pb, polar body. A bright-field image is shown on the far left. (B) Two-cell–stage embryos double-stained with anti-5hmC antibody (green) and anti-5mC antibody (red). These images were obtained by confocal microscopy. (C) Confocal microscopy image of a two-cell (2c) stage embryo entering mitosis. The condensed chromosomes are labeled with anti-5mC antibody (red) and anti-5hmC antibody (green). (D) 5hmC and 5mC in four- (4c) and eight-cell (8c) –stage embryos. Four-cell (Upper Left) and eight-cell (remaining images) embryos were double-stained with anti-5hmC antibody (green) and anti-5mC antibody (red). A confocal image is shown in the upper right image.
Fig. 4.
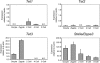
Expression of Tet and Stella/Dppa3 genes in oocytes, zygotes, and early cleavage-stage embryos. RNA was isolated from oocytes, zygotes, two-, four-, and eight-cell–stage embryos. Real-time PCR was used to assess the expression of the three Tet genes and Stella/Dppa3. Data were normalized relative to expression of β-actin. N.D., no detectable signal in real-time PCR. Expression of Tet1 in the zygote and of Tet3 at the two-cell stage has a detectable signal, which is close to zero.
Fig. 5.
Sodium bisulfite sequencing of Line1, ETn, Mylc, and Acta1 sequences in sperm, oocytes, and zygotes. DNA was isolated from mouse oocytes, sperm, or zygotes (PN4–PN5) and subjected to sodium bisulfite conversion. (A) Line1 5′ end sequences were amplified, cloned, and sequenced. Open squares, unmethylated CpGs; black squares, methylated CpGs; gray squares, not analyzable/mutated CpG site. Each row represents an individual sequenced DNA strand. (B) ETn sequences were amplified, cloned, and sequenced. The sequences from zygotes represent the paternal allele distinguishable by a sequence polymorphism. (C) Acta1 sequences. (D) Myl3 sequences. The percentage of methylated CpGs is indicated.
Similar articles
- 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming.
Wossidlo M, Nakamura T, Lepikhov K, Marques CJ, Zakhartchenko V, Boiani M, Arand J, Nakano T, Reik W, Walter J. Wossidlo M, et al. Nat Commun. 2011;2:241. doi: 10.1038/ncomms1240. Nat Commun. 2011. PMID: 21407207 - The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes.
Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, Xie ZG, Shi L, He X, Jin SG, Iqbal K, Shi YG, Deng Z, Szabó PE, Pfeifer GP, Li J, Xu GL. Gu TP, et al. Nature. 2011 Sep 4;477(7366):606-10. doi: 10.1038/nature10443. Nature. 2011. PMID: 21892189 - Comparative dynamics of 5-methylcytosine reprogramming and TET family expression during preimplantation mammalian development in mouse and sheep.
Jafarpour F, Hosseini SM, Ostadhosseini S, Abbasi H, Dalman A, Nasr-Esfahani MH. Jafarpour F, et al. Theriogenology. 2017 Feb;89:86-96. doi: 10.1016/j.theriogenology.2016.10.010. Epub 2016 Oct 20. Theriogenology. 2017. PMID: 28043375 - 5-Hydroxymethylcytosine: a stable or transient DNA modification?
Hahn MA, Szabó PE, Pfeifer GP. Hahn MA, et al. Genomics. 2014 Nov;104(5):314-23. doi: 10.1016/j.ygeno.2014.08.015. Epub 2014 Aug 30. Genomics. 2014. PMID: 25181633 Free PMC article. Review. - Genomic distribution and possible functions of DNA hydroxymethylation in the brain.
Wen L, Tang F. Wen L, et al. Genomics. 2014 Nov;104(5):341-6. doi: 10.1016/j.ygeno.2014.08.020. Epub 2014 Sep 7. Genomics. 2014. PMID: 25205307 Review.
Cited by
- Transcriptional activation of transposable elements in mouse zygotes is independent of Tet3-mediated 5-methylcytosine oxidation.
Inoue A, Matoba S, Zhang Y. Inoue A, et al. Cell Res. 2012 Dec;22(12):1640-9. doi: 10.1038/cr.2012.160. Epub 2012 Nov 27. Cell Res. 2012. PMID: 23184059 Free PMC article. - Loss of UHRF2 expression is associated with human neoplasia, promoter hypermethylation, decreased 5-hydroxymethylcytosine, and high proliferative activity.
Lu H, Bhoopatiraju S, Wang H, Schmitz NP, Wang X, Freeman MJ, Forster CL, Verneris MR, Linden MA, Hallstrom TC. Lu H, et al. Oncotarget. 2016 Nov 15;7(46):76047-76061. doi: 10.18632/oncotarget.12583. Oncotarget. 2016. PMID: 27738314 Free PMC article. - Recognition and potential mechanisms for replication and erasure of cytosine hydroxymethylation.
Hashimoto H, Liu Y, Upadhyay AK, Chang Y, Howerton SB, Vertino PM, Zhang X, Cheng X. Hashimoto H, et al. Nucleic Acids Res. 2012 Jun;40(11):4841-9. doi: 10.1093/nar/gks155. Epub 2012 Feb 22. Nucleic Acids Res. 2012. PMID: 22362737 Free PMC article. - Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers.
Seisenberger S, Peat JR, Hore TA, Santos F, Dean W, Reik W. Seisenberger S, et al. Philos Trans R Soc Lond B Biol Sci. 2013 Jan 5;368(1609):20110330. doi: 10.1098/rstb.2011.0330. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23166394 Free PMC article. Review. - DNA methylation and methylcytosine oxidation in cell fate decisions.
Koh KP, Rao A. Koh KP, et al. Curr Opin Cell Biol. 2013 Apr;25(2):152-61. doi: 10.1016/j.ceb.2013.02.014. Epub 2013 Mar 14. Curr Opin Cell Biol. 2013. PMID: 23498662 Free PMC article. Review.
References
- Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187:226–232. - PubMed
- Riggs AD. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14:9–25. - PubMed
- Hochedlinger K, Jaenisch R. Nuclear reprogramming and pluripotency. Nature. 2006;441:1061–1067. - PubMed
- Huang K, Fan G. DNA methylation in cell differentiation and reprogramming: An emerging systematic view. Regen Med. 2010;5:531–544. - PubMed
- Straussman R, et al. Developmental programming of CpG island methylation profiles in the human genome. Nat Struct Mol Biol. 2009;16:564–571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM064378/GM/NIGMS NIH HHS/United States
- R01 AG036041/AG/NIA NIH HHS/United States
- ES015185/ES/NIEHS NIH HHS/United States
- R01 ES015185/ES/NIEHS NIH HHS/United States
- GM064378/GM/NIGMS NIH HHS/United States
- AG036041/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases