Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity - PubMed (original) (raw)
Randomized Controlled Trial
. 2011 Feb 17;364(7):603-15.
doi: 10.1056/NEJMoa1007374.
Collaborators, Affiliations
- PMID: 21323540
- PMCID: PMC3119530
- DOI: 10.1056/NEJMoa1007374
Randomized Controlled Trial
Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity
Helen A Mintz-Hittner et al. N Engl J Med. 2011.
Abstract
Background: Retinopathy of prematurity is a leading cause of childhood blindness worldwide. Peripheral retinal ablation with conventional (confluent) laser therapy is destructive, causes complications, and does not prevent all vision loss, especially in cases of retinopathy of prematurity affecting zone I of the eye. Case series in which patients were treated with vascular endothelial growth factor inhibitors suggest that these agents may be useful in treating retinopathy of prematurity.
Methods: We conducted a prospective, controlled, randomized, stratified, multicenter trial to assess intravitreal bevacizumab monotherapy for zone I or zone II posterior stage 3+ (i.e., stage 3 with plus disease) retinopathy of prematurity. Infants were randomly assigned to receive intravitreal bevacizumab (0.625 mg in 0.025 ml of solution) or conventional laser therapy, bilaterally. The primary ocular outcome was recurrence of retinopathy of prematurity in one or both eyes requiring retreatment before 54 weeks' postmenstrual age.
Results: We enrolled 150 infants (total sample of 300 eyes); 143 infants survived to 54 weeks' postmenstrual age, and the 7 infants who died were not included in the primary-outcome analyses. Retinopathy of prematurity recurred in 4 infants in the bevacizumab group (6 of 140 eyes [4%]) and 19 infants in the laser-therapy group (32 of 146 eyes [22%], P=0.002). A significant treatment effect was found for zone I retinopathy of prematurity (P=0.003) but not for zone II disease (P=0.27).
Conclusions: Intravitreal bevacizumab monotherapy, as compared with conventional laser therapy, in infants with stage 3+ retinopathy of prematurity showed a significant benefit for zone I but not zone II disease. Development of peripheral retinal vessels continued after treatment with intravitreal bevacizumab, but conventional laser therapy led to permanent destruction of the peripheral retina. This trial was too small to assess safety. (Funded by Research to Prevent Blindness and others; ClinicalTrials.gov number, NCT00622726.).
Figures
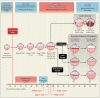
Figure 1. Pathogenesis and Therapy of Retinopathy of Prematurity (ROP)
Phases 1 and 2 of ROP are associated with different levels of vascular endothelial growth factor (VEGF), oxygen, and neovascular activity. ROP stages 0 to 5 are shown, as are the outcomes, when therapy is successful, of cryotherapy (established in a clinical trial in 1988), laser therapy (established in clinical trial in 2003), and intravitreal bevacizumab (this study). Cryotherapy involves scarring of the full ocular thickness, laser therapy scarring of the retinal thickness, and intravitreal bevacizumab scarring with a needle near the limbus. Also shown are the postmenstrual ages at which infants are at high risk for ROP, the appropriate postmenstrual age for the first ocular screening examination for ROP, and the mean postmenstrual ages at the onset of aggressive posterior ROP (APROP) (type 2 ROP) and stages 1, 2, and 3 (type 1 ROP).
Figure 2
Enrollment, Randomization, and Follow-up of the 150 Study Infants.
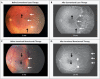
Figure 3. Fundus Photographs and Fluorescein Angiograms of Retinas in Study Infants with Stage 3+ Retinopathy of Prematurity in Zone I, before and after Treatment
Panels A and B show the left retina of an infant before conventional laser therapy (at approximately 2 months of age, or 33.1 weeks’ postmenstrual age) and after therapy (at 13 months’ postmenstrual age), respectively. The infant was born at 24 weeks’ gestational age, with a birth weight of 760 g. The post-treatment photograph shows destruction of the full thickness of the peripheral retina, with only choroidal vessels (not retinal vessels) visible in the lasered area. Panels C and D show the left retina in another infant before intravitreal bevacizumab therapy (at approximately 3 months of age, or 35.6 weeks’ postmenstrual age) and after therapy (at 13 months’ postmenstrual age), respectively. The infant was born at 23 weeks’ gestational age, with a birth weight of 495 g. The post-treatment photograph shows continued vascularization of the peripheral retina. In all four panels, black arrows indicate identical retinal points for comparison before and after treatment, and thin white arrows indicate the extent of vascularization at each time point; the wide white arrows in Panel D indicate the extent of vascularization at the time of treatment with bevacizumab.
Comment in
- Bevacizumab for retinopathy of prematurity.
Reynolds JD. Reynolds JD. N Engl J Med. 2011 Feb 17;364(7):677-8. doi: 10.1056/NEJMe1100248. N Engl J Med. 2011. PMID: 21323546 No abstract available. - Bevacizumab for retinopathy of prematurity.
Lim LS, Mitchell P, Wong TY. Lim LS, et al. N Engl J Med. 2011 Jun 16;364(24):2360; author reply 2361-2. doi: 10.1056/NEJMc1103460. N Engl J Med. 2011. PMID: 21675897 No abstract available. - Bevacizumab for retinopathy of prematurity.
Gilbert CE, Zin A, Darlow B. Gilbert CE, et al. N Engl J Med. 2011 Jun 16;364(24):2359-60; author reply 2361-2. doi: 10.1056/NEJMc1103460. N Engl J Med. 2011. PMID: 21675898 No abstract available. - Bevacizumab for retinopathy of prematurity.
Good WV, Palmer EA. Good WV, et al. N Engl J Med. 2011 Jun 16;364(24):2359; author reply 2361-2. doi: 10.1056/NEJMc1103460. N Engl J Med. 2011. PMID: 21675899 No abstract available.
Similar articles
- [Effects of intravitreal pegaptanib or bevacizumab and laser in treatment of threshold retinopathy of prematurity in zone I and posterior zone II--four years results].
Autrata R, Senková K, Holousová M, Krejcírová I, Dolezel Z, Borek I. Autrata R, et al. Cesk Slov Oftalmol. 2012 Feb;68(1):29-36. Cesk Slov Oftalmol. 2012. PMID: 22679695 Clinical Trial. Czech. - Efficacy of intravitreal bevacizumab for zone-II retinopathy of prematurity.
Karkhaneh R, Khodabande A, Riazi-Eafahani M, Roohipoor R, Ghassemi F, Imani M, Dastjani Farahani A, Ebrahimi Adib N, Torabi H. Karkhaneh R, et al. Acta Ophthalmol. 2016 Sep;94(6):e417-20. doi: 10.1111/aos.13008. Epub 2016 Mar 24. Acta Ophthalmol. 2016. PMID: 27009449 Clinical Trial. - Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: a randomized clinical trial.
Geloneck MM, Chuang AZ, Clark WL, Hunt MG, Norman AA, Packwood EA, Tawansy KA, Mintz-Hittner HA; BEAT-ROP Cooperative Group. Geloneck MM, et al. JAMA Ophthalmol. 2014 Nov;132(11):1327-33. doi: 10.1001/jamaophthalmol.2014.2772. JAMA Ophthalmol. 2014. PMID: 25103848 Clinical Trial. - Antivascular endothelial growth factor for retinopathy of prematurity.
Mintz-Hittner HA, Best LM. Mintz-Hittner HA, et al. Curr Opin Pediatr. 2009 Apr;21(2):182-7. doi: 10.1097/MOP.0b013e32832925f9. Curr Opin Pediatr. 2009. PMID: 19300261 Review. - [Off-label use of intravitreal bevacizumab for severe retinopathy of prematurity].
Alba LE, Zaldua RA, Masini RA. Alba LE, et al. Arch Soc Esp Oftalmol. 2015 Feb;90(2):81-6. doi: 10.1016/j.oftal.2014.09.011. Epub 2014 Nov 15. Arch Soc Esp Oftalmol. 2015. PMID: 25459682 Review. Spanish.
Cited by
- Treatment of aggressive posterior retinopathy of prematurity accompanied by nasolacrimal duct obstruction with purulent discharge: A case report.
Namvar E, Attar A. Namvar E, et al. Clin Case Rep. 2024 Nov 5;12(11):e9533. doi: 10.1002/ccr3.9533. eCollection 2024 Nov. Clin Case Rep. 2024. PMID: 39502123 Free PMC article. - Ten-year outcomes after initial management with laser photocoagulation versus intravitreal bevacizumab injection in a pair of identical twins with aggressive posterior retinopathy of prematurity.
Jeon SH, Roh YJ. Jeon SH, et al. Am J Ophthalmol Case Rep. 2021 May 2;22:101097. doi: 10.1016/j.ajoc.2021.101097. eCollection 2021 Jun. Am J Ophthalmol Case Rep. 2021. PMID: 34027226 Free PMC article. - Incidence, Long-Term Visual Outcomes, and Mortality in Retinopathy of Prematurity in Korea: A Nationwide Population-Based Study.
Na KH, Kim KH, Kang TU, Hann HJ, Ahn HS, Kim HJ. Na KH, et al. Invest Ophthalmol Vis Sci. 2020 Aug 3;61(10):14. doi: 10.1167/iovs.61.10.14. Invest Ophthalmol Vis Sci. 2020. PMID: 32761140 Free PMC article. - Intravitreally Injected Anti-VEGF Antibody Reduces Brown Fat in Neonatal Mice.
Jo DH, Park SW, Cho CS, Powner MB, Kim JH, Fruttiger M, Kim JH. Jo DH, et al. PLoS One. 2015 Jul 30;10(7):e0134308. doi: 10.1371/journal.pone.0134308. eCollection 2015. PLoS One. 2015. PMID: 26226015 Free PMC article. - Comparison in Retreatments between Bevacizumab and Ranibizumab Intravitreal Injections for Retinopathy of Prematurity: A Multicenter Study.
Patel NA, Acaba-Berrocal LA, Hoyek S, Fan KC, Martinez-Castellanos MA, Baumal CR, Harper CA 3rd, Berrocal AM; Retinopathy of Prematurity Injection Consortium (ROPIC). Patel NA, et al. Ophthalmology. 2023 Apr;130(4):373-378. doi: 10.1016/j.ophtha.2022.11.012. Epub 2022 Nov 15. Ophthalmology. 2023. PMID: 36396121 Free PMC article.
References
- Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82. - PubMed
- Katz X, Kychenthal A, Dorta P. Zone I retinopathy of prematurity. J AAPOS. 2000;4:373–6. - PubMed
- O’Keefe M, Lanigan B, Long VW. Outcomes of zone I retinopathy of prematurity. Acta Ophthalmol Scand. 2003;81:614–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 EY010608-17/EY/NEI NIH HHS/United States
- UL1 RR024148-05/RR/NCRR NIH HHS/United States
- UL1 RR024148/RR/NCRR NIH HHS/United States
- P30 EY010608/EY/NEI NIH HHS/United States
- P30EY10608/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources