Principles and current strategies for targeting autophagy for cancer treatment - PubMed (original) (raw)
Review
Principles and current strategies for targeting autophagy for cancer treatment
Ravi K Amaravadi et al. Clin Cancer Res. 2011.
Abstract
Autophagy is an evolutionarily conserved, intracellular self-defense mechanism in which organelles and proteins are sequestered into autophagic vesicles that are subsequently degraded through fusion with lysosomes. Cells, thereby, prevent the toxic accumulation of damaged or unnecessary components, but also recycle these components to sustain metabolic homoeostasis. Heightened autophagy is a mechanism of resistance for cancer cells faced with metabolic and therapeutic stress, revealing opportunities for exploitation as a therapeutic target in cancer. We summarize recent developments in the field of autophagy and cancer and build upon the results presented at the Cancer Therapy Evaluation Program (CTEP) Early Drug Development meeting in March 2010. Herein, we describe our current understanding of the core components of the autophagy machinery and the functional relevance of autophagy within the tumor microenvironment, and we outline how this knowledge has informed preclinical investigations combining the autophagy inhibitor hydroxychloroquine (HCQ) with chemotherapy, targeted therapy, and immunotherapy. Finally, we describe ongoing clinical trials involving HCQ as a first generation autophagy inhibitor, as well as strategies for the development of novel, more potent, and specific inhibitors of autophagy.
©2011 AACR.
Figures
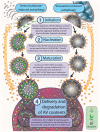
Figure 1. Anatomy of autophagy
Autophagy occurs through a multi-step process including 4 control points: 1) initiation, 2) nucleation, 3) maturation, and 4) delivery and degradation of AV contents. These steps occur irrespective of whether autophagy has been induced through stress/ubiquitinated substrate accumulation, or through starvation. During initiation, nascent AV membranes derived from multiple potential sources (including isolated membranes, ER or mitochondria outer membranes) form a cup-like structure onto which autophagosomal machinery, including LC3, dynamically associates. As the cup-like structure enlarges, it sequesters substrate, which includes ubiquitinated proteins or organelles in the case of stress/substrate induced autophagy, and soluble cytoplasm in the case of starvation induced autophagy. The double membrane comprising the nascent AV then closes to form the mature autophagic vesicle, which then targets and fuses with the lysosome. In the lysosome, hydrolytic enzymes digest the contents and inner membrane of the AV, with autophagic machinery (i.e., LC3) recycled through the cytoplasm for recruitment to other nascent autophagosomes.
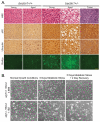
Figure 2. Role of autophagy in suppressing liver damage and cell death
(A) Elevated p62, ubiquitin and accumulation of lipids in aged beclin1+/− mouse liver. Sections of liver from young (16 months old) and aged (> 24 months old) beclin1 +/+ and +/− mice and a representative spontaneous liver tumor from a beclin1+/− mouse were stained with H&E, and by immunohistochemistry for p62 and ubiquitin, and with BODIPY to indicate lipid droplet accumulation by fluorescence. (B) Autophagy promotes cell viability in metabolic stress. Representative images of immortal baby mouse kidney epithelial (iBMK) cells derived from atg7+/+ and −/− mice that were untreated, treated with metabolic stress (ischemia: no glucose and 1% oxygen or hypoxic conditions) and allowed to recover (35). Images generously provided by Dr. C. Karp and H.-Y. Chen from the White laboratory.
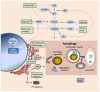
Figure 3. Network interactions between kinase signaling, protein metabolism and autophagy
Metabolic and therapeutic stresses (green) converge on mTORC1 and mediators of ER stress to induce autophagy. Autophagy serves a cytoprotective role in response to these stresses, by clearing damaged organelles, aggregated proteins, and recycling macromolecules to sustain survival. Arrows:activation; flat lines: inhibition. PI3K: phosphotidyl inositol-3 kinase IRS1: insulin receptor substrate 1; mTORC2: mammalian target of rapamycin complex 2; TSC: tuberous sclerosis complex; AMPK: AMP-activated protein kinase; REDD1: regulated in development and DNA damage 1; mTORC1: mammalian target of rapamycin complex 1; PERK: protein kinase-like endoplasmic reticulum kinase; eIF2α: eukaryotic initiation factor 2α: ER: endoplasmic reticulum; IRE-1: inositol requiring enzyme-1; JNK: Jun N-terminal kinase; HCQ: hydroxychloroquine; PI3K/mTORi: dual PI3K/mTOR inhibitors
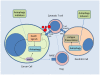
Figure 4. Cancer Immunity and autophagy
Imbalance of autophagy leads to immune tolerance in cancer patients. Heightened autophagy in tumor cells prevents immune effector cell mediated cytotoxicity. In addition, Stress-induced release of the damage-associated molecular pattern molecule HMGB1 induces cytoprotective autophagy and once extruded into the extracellular matrix recruits regulatory T cells (Treg) resulting in anergy. Suppressed autophagy in dendrtic cells limits effective priming of antigen presentation that trains cytotoxic T cells. Simultaneous or sequential pharmacological induction of autophagy in dendritic cells, and autophagy inhibition in the tumor cell would ideally reverse this imbalance and enhance antitumor immunity. FAS: FS7-associated cell surface antigen; FASL: Fas ligand; MHC: major histocompatability complex; RAGE: receptor for advanced glycation endproducts
Figure 5. Pharmacodynamic assay for autophagy inhibition
Electron micrographs of peripheral blood mononuclear cells from a glioma patient enrolled on the phase I trial of temozolomide, radiation and hydroxychloroquine; (A) Pretreatment and (B) 3 weeks of combined therapy. Arrows: autophagic vesicles, scale bar 200 μm.
Similar articles
- Targeting Autophagy in Cancer: Update on Clinical Trials and Novel Inhibitors.
Chude CI, Amaravadi RK. Chude CI, et al. Int J Mol Sci. 2017 Jun 16;18(6):1279. doi: 10.3390/ijms18061279. Int J Mol Sci. 2017. PMID: 28621712 Free PMC article. Review. - The role of autophagy in cancer: therapeutic implications.
Yang ZJ, Chee CE, Huang S, Sinicrope FA. Yang ZJ, et al. Mol Cancer Ther. 2011 Sep;10(9):1533-41. doi: 10.1158/1535-7163.MCT-11-0047. Epub 2011 Aug 30. Mol Cancer Ther. 2011. PMID: 21878654 Free PMC article. Review. - Induction of autophagy is an early response to gefitinib and a potential therapeutic target in breast cancer.
Dragowska WH, Weppler SA, Wang JC, Wong LY, Kapanen AI, Rawji JS, Warburton C, Qadir MA, Donohue E, Roberge M, Gorski SM, Gelmon KA, Bally MB. Dragowska WH, et al. PLoS One. 2013 Oct 11;8(10):e76503. doi: 10.1371/journal.pone.0076503. eCollection 2013. PLoS One. 2013. PMID: 24146879 Free PMC article. - Combined MTOR and autophagy inhibition: phase I trial of hydroxychloroquine and temsirolimus in patients with advanced solid tumors and melanoma.
Rangwala R, Chang YC, Hu J, Algazy KM, Evans TL, Fecher LA, Schuchter LM, Torigian DA, Panosian JT, Troxel AB, Tan KS, Heitjan DF, DeMichele AM, Vaughn DJ, Redlinger M, Alavi A, Kaiser J, Pontiggia L, Davis LE, O'Dwyer PJ, Amaravadi RK. Rangwala R, et al. Autophagy. 2014 Aug;10(8):1391-402. doi: 10.4161/auto.29119. Epub 2014 May 20. Autophagy. 2014. PMID: 24991838 Free PMC article. Clinical Trial. - Recent advances in targeting autophagy in cancer.
Jain V, Singh MP, Amaravadi RK. Jain V, et al. Trends Pharmacol Sci. 2023 May;44(5):290-302. doi: 10.1016/j.tips.2023.02.003. Epub 2023 Mar 15. Trends Pharmacol Sci. 2023. PMID: 36931971 Free PMC article. Review.
Cited by
- LC3B Binds to the Autophagy Protease ATG4b with High Affinity Using a Bipartite Interface.
Tang Y, Kay A, Jiang Z, Arkin MR. Tang Y, et al. Biochemistry. 2022 Nov 1;61(21):2295-2302. doi: 10.1021/acs.biochem.2c00482. Epub 2022 Oct 20. Biochemistry. 2022. PMID: 36264309 Free PMC article. - Calhex231 ameliorates myocardial fibrosis post myocardial infarction in rats through the autophagy-NLRP3 inflammasome pathway in macrophages.
Liu W, Sun J, Guo Y, Liu N, Ding X, Zhang X, Chi J, Kang N, Liu Y, Yin X. Liu W, et al. J Cell Mol Med. 2020 Nov;24(22):13440-13453. doi: 10.1111/jcmm.15969. Epub 2020 Oct 12. J Cell Mol Med. 2020. PMID: 33043596 Free PMC article. - The role for autophagy in cancer.
White E. White E. J Clin Invest. 2015 Jan;125(1):42-6. doi: 10.1172/JCI73941. Epub 2015 Jan 2. J Clin Invest. 2015. PMID: 25654549 Free PMC article. Review. - Autophagy modulation as a target for anticancer drug discovery.
Li X, Xu HL, Liu YX, An N, Zhao S, Bao JK. Li X, et al. Acta Pharmacol Sin. 2013 May;34(5):612-24. doi: 10.1038/aps.2013.23. Epub 2013 Apr 8. Acta Pharmacol Sin. 2013. PMID: 23564085 Free PMC article. Review. - Repositioning of Antiparasitic Drugs for Tumor Treatment.
Li YQ, Zheng Z, Liu QX, Lu X, Zhou D, Zhang J, Zheng H, Dai JG. Li YQ, et al. Front Oncol. 2021 Apr 29;11:670804. doi: 10.3389/fonc.2021.670804. eCollection 2021. Front Oncol. 2021. PMID: 33996598 Free PMC article. Review.
References
- Kuma A, Mizushima N. Physiological role of autophagy as an intracellular recycling system: with an emphasis on nutrient metabolism. Semin Cell Dev Biol. 21:683–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1K23CA120862-01A2/CA/NCI NIH HHS/United States
- RC1 CA147961/CA/NCI NIH HHS/United States
- CA111456/CA/NCI NIH HHS/United States
- R01CA130893/CA/NCI NIH HHS/United States
- K23 CA120862/CA/NCI NIH HHS/United States
- RC1CA147961/CA/NCI NIH HHS/United States
- P50 CA097257/CA/NCI NIH HHS/United States
- RC1 CA147961-02/CA/NCI NIH HHS/United States
- R01 CA130893-04/CA/NCI NIH HHS/United States
- CA83817/CA/NCI NIH HHS/United States
- R37 CA053370/CA/NCI NIH HHS/United States
- R01 CA083817/CA/NCI NIH HHS/United States
- R37 CA053370-19/CA/NCI NIH HHS/United States
- U01 CA132194/CA/NCI NIH HHS/United States
- P50CA097257/CA/NCI NIH HHS/United States
- R37CA53370/CA/NCI NIH HHS/United States
- R01 CA130893/CA/NCI NIH HHS/United States
- R01 CA111456/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources