Dorsal radial glial cells have the potential to generate cortical interneurons in human but not in mouse brain - PubMed (original) (raw)
Dorsal radial glial cells have the potential to generate cortical interneurons in human but not in mouse brain
Xiaojing Yu et al. J Neurosci. 2011.
Abstract
Radial glial (RG) cells, in the neocortical ventricular/subventricular zone (VZ/SVZ), generate cortical projection neurons both in rodents and humans, but whether they can also generate cortical interneurons is not clear. We demonstrated both on cryosections and in cell cultures that in the human VZ/SVZ, cells can be double labeled with RG markers and calretinin (CalR) and GABA, markers that suggest interneuronal lineage. We examined in more detail the cell fate of human RG cells isolated from the VZ/SVZ at midterm. After 24 h, no CalR(+) or GABA(+) cells were seen in cultures, whereas 5-10% cells expressed Nkx2.1 and Dlx, two ventral transcription factors. CalR(+) and GABA(+) cells were apparent for the first time after 3 d in vitro, and their number increased in subsequent days, consistent with the gradual transition of RG cells into CalR(+) or GABA(+) cells. Indeed, the progeny of genetically labeled RG cells could be immunolabeled with antibodies to CalR and GABA or ventral transcription factors (Nkx2.1(+), Dlx(+)). In contrast to humans, in the embryonic mouse, similar experiments showed that only RG cells isolated from the subpallium (ganglionic eminence) generate CalR(+) or GABA(+) cells, whereas this was not the case with RG cells isolated from the pallium. These findings support the idea that human, but not mouse, dorsal RG cells have the potential to generate various subtypes of neocortical interneurons. Multiple progenitors and sites of cortical interneuron origin in human might be an evolutionary adaptation underlying brain expansion and the increased complexity of cortical circuitry in humans.
Figures
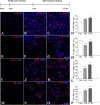
Figure 1.
The percentages of cells labeled with interneuronal markers CalR and GABA and ventral transcription factors Nkx2.1 and Dlx from human VZ/SVZ increase over time. The top diagram shows the time scale for in vitro experiments with proliferation (10 ng/ml bFGF) and differentiation (without bFGF) media. Representative images of CalR+, GABA+, Nkx2.1+, and Dlx+ cells after 4 h (A, E, I, M) and 5 div (B, F, J, N) in proliferation medium and after additional 7 div in differentiation medium (C, G, K, O). Graphs show corresponding percentages over time (D, H, L, P). Blue-nuclear bisbenzamide staining was used. *p ≤ 0.05; **p ≤ 0.001. Scale bars, A–O, 20 μm.
Figure 2.
Human and mouse cryosections and cultures. A–C, Immunofluorescence of cryosections at 20 g.w. fetal brain vimentin (Vim; red) and Nkx2.1 (green) in the cortical VZ/SVZ. Inset, Higher magnification of colabeled cell. D, In the coronal section of the E16 mouse forebrain, there is a strong Nkx2.1 expression (green) in the subpallium but not in the pallium. LGE, Llateral GE; LV, lateral ventricle; Sep, septal area; Str, striatum. E, F, Higher magnification of the cortex (E) and MGE (F). G, In acute cell culture (4 h), cells isolated from the human cortical VZ/SVZ at midterm express Nkx2.1 (red). H, I, In contrast, in E16 mouse cell culture from the pallium (H), Nkx2.1 is not demonstrated, but there are numerous Nkx2.1+ cells in the subpallium culture (I, green and arrows). The dashed line in A–C represents the ventricular surface. BB, Blue bizbenzamide nuclear stain. Scale bars: A–C, G–I, 20 μm; inset, 10 μm; D–F, 50 μm.
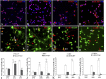
Figure 3.
Human Lex+ cells coexpress markers for radial glia and interneurons in the same cells in vitro. A–D, After 24 h in the proliferation medium, the majority of LeX+ cells are colabeled with one of the RG markers: Pax6 (A), vimentin (Vim; B), GFAP (C), or BLBP (D). E–H, After 3 div, representative images of cells double labeled with Nkx2.1 and Vim (E), Dlx and Vim (F), CalR and BLBP (G), and GABA and Vim (H) are shown. I–L, Graphs demonstrate the proportion of single- and double-labeled cells from all the cells in the above illustrated cultures after 24 h in PM and after 3 and 7 div in DM. Scale bars, A–H, 20 μm. *p < 0.05; **p < 0.001.
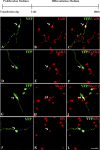
Figure 4.
A fraction of BLBP-Cre LoxP-transfected human RG cells generate cells of the interneuron lineage after 7 div differentiation: A–C, CalR (red, arrow); D–F, GABA (red, arrow); G–I, Nkx2.1+ (red, arrow); J–L, Dlx+ (red, arrow) cells. Scale bar: (in L) A–L, 20 μm. The top diagram shows the time scale for particular in vitro experiments with proliferation (10 ng/ml bFGF) and differentiation (without bFGF) medium.
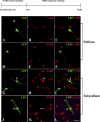
Figure 5.
Mouse RG cells isolated from the forebrain at E16, transfected with BLBP-Cre LoxP and differentiated for 7 div. A–F, RG from the pallium (dorsal telencephalon) do not generate cells labeled with either CalR (A–C, red) or GABA (D–F, red). G–L, In contrast, RG cells from the subpallium (ventral telencephalon) generate cells in the interneuron lineage, CalR+ (G–I, red, arrow) and GABA+ (J–L, red, arrow) cells. Scale bar: (in L) A–L, 20 μm. The top diagram shows the time scale for particular in vitro experiments with proliferation (10 ng/ml bFGF) and differentiation (without bFGF) medium.
Similar articles
- Ventralized dorsal telencephalic progenitors in Pax6 mutant mice generate GABA interneurons of a lateral ganglionic eminence fate.
Kroll TT, O'Leary DD. Kroll TT, et al. Proc Natl Acad Sci U S A. 2005 May 17;102(20):7374-9. doi: 10.1073/pnas.0500819102. Epub 2005 May 6. Proc Natl Acad Sci U S A. 2005. PMID: 15878992 Free PMC article. - Multiple origins of human neocortical interneurons are supported by distinct expression of transcription factors.
Jakovcevski I, Mayer N, Zecevic N. Jakovcevski I, et al. Cereb Cortex. 2011 Aug;21(8):1771-82. doi: 10.1093/cercor/bhq245. Epub 2010 Dec 7. Cereb Cortex. 2011. PMID: 21139075 Free PMC article. - Extended Production of Cortical Interneurons into the Third Trimester of Human Gestation.
Arshad A, Vose LR, Vinukonda G, Hu F, Yoshikawa K, Csiszar A, Brumberg JC, Ballabh P. Arshad A, et al. Cereb Cortex. 2016 May;26(5):2242-2256. doi: 10.1093/cercor/bhv074. Epub 2015 Apr 16. Cereb Cortex. 2016. PMID: 25882040 Free PMC article. - Molecules and mechanisms involved in the generation and migration of cortical interneurons.
Hernández-Miranda LR, Parnavelas JG, Chiara F. Hernández-Miranda LR, et al. ASN Neuro. 2010 Mar 31;2(2):e00031. doi: 10.1042/AN20090053. ASN Neuro. 2010. PMID: 20360946 Free PMC article. Review. - Cortical interneuron fate determination: diverse sources for distinct subtypes?
Xu Q, de la Cruz E, Anderson SA. Xu Q, et al. Cereb Cortex. 2003 Jun;13(6):670-6. doi: 10.1093/cercor/13.6.670. Cereb Cortex. 2003. PMID: 12764043 Review.
Cited by
- The Principle of Cortical Development and Evolution.
Yang Z. Yang Z. Neurosci Bull. 2024 Jul 18. doi: 10.1007/s12264-024-01259-2. Online ahead of print. Neurosci Bull. 2024. PMID: 39023844 Review. - A molecular and cellular perspective on human brain evolution and tempo.
Lindhout FW, Krienen FM, Pollard KS, Lancaster MA. Lindhout FW, et al. Nature. 2024 Jun;630(8017):596-608. doi: 10.1038/s41586-024-07521-x. Epub 2024 Jun 19. Nature. 2024. PMID: 38898293 Review. - Hedgehog Signaling in Cortical Development.
Cai E, Barba MG, Ge X. Cai E, et al. Cells. 2023 Dec 21;13(1):21. doi: 10.3390/cells13010021. Cells. 2023. PMID: 38201225 Free PMC article. Review. - Neurogenesis in primates versus rodents and the value of non-human primate models.
Zhang R, Quan H, Wang Y, Luo F. Zhang R, et al. Natl Sci Rev. 2023 Sep 15;10(11):nwad248. doi: 10.1093/nsr/nwad248. eCollection 2023 Nov. Natl Sci Rev. 2023. PMID: 38025664 Free PMC article. Review. - A facile method to generate cerebral organoids from human pluripotent stem cells.
Simorgh S, Mousavi SA, To SK, Pasque V, Wierda K, Vervliet T, Yeganeh M, Pooyan P, Chai YC, Verfaillie C, Baharvand H. Simorgh S, et al. EXCLI J. 2023 Oct 5;22:1055-1076. doi: 10.17179/excli2023-6299. eCollection 2023. EXCLI J. 2023. PMID: 37927348 Free PMC article.
References
- Alcantara S, de Lecea L, Del Rio JA, Ferrer I, Soriano E. Transient colocalization of parvalbumin and calbindin D28k in the postnatal cerebral cortex: evidence for a phenotypic shift in developing nonpyramidal neurons. Eur J Neurosci. 1996;8:1329–1339. - PubMed
- Anderson S, Mione M, Yun K, Rubenstein JL. Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex. 1999;9:646–654. - PubMed
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. - PubMed
- Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous