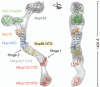Characterization of the membrane-coating Nup84 complex: paradigm for the nuclear pore complex structure - PubMed (original) (raw)
Review
Characterization of the membrane-coating Nup84 complex: paradigm for the nuclear pore complex structure
Erik W Debler et al. Nucleus. 2010 Mar-Apr.
Abstract
Nuclear pore complexes (NPCs) function as selective gates for nucleocytoplasmic transport. Although the NPC was discovered more than half a century ago, our knowledge of NPC components in atomic detail has exploded only over the past few years. Recent structural, biochemical, and in vivo studies of NPC components, in particular the membrane-coating heptameric Nup84 complex, have shed light onto the NPC architecture as well as onto its dynamic nature. Striking similarities were revealed between the components of the NPC and of coat protein complexes in the endocytic and secretory pathways, supporting their common evolutionary origin in a progenitor protocoatomer. Here, we summarize these findings and discuss emerging concepts that underlie the molecular architecture and the dynamics of the NPC. We conclude that the uncovered principles are not limited to the NPC, but are likely to extend to other macromolecular assemblies.
Keywords: binding promiscuity; coat protein complex; coatomer; crystallography; dynamic interaction; electron microscopy; macromolecular assembly; nucleocytoplasmic transport; nucleoporin; vesicle coats.
Figures
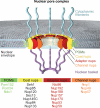
Figure 1
Transport functions of the NPC. (A) Overall, the NPC consists of a cylindrical, symmetric core, which is asymmetrically decorated with filaments and a nuclear basket structure on the cytoplasmic and nucleoplasmic sides, respectively. Molecules smaller than ∼40 kDa freely diffuse through the NPC. (B) Active import and export of cargoes are facilitated by nuclear localization and nuclear export sequences (NLS and NES, respectively) that are recognized by transport factors, collectively termed karyopherins (kaps). The NLS of import cargoes is recognized either directly by an import karyopherin-β (kap-β) or via an adapter karyopherin (kap-α). Ran•GTP binding inside the nucleus leads to dissociation of the import complex (left). By contrast, the assembly of an NES-cargo kap-β export complex requires Ran•GTP binding. In the cytosol, this export complex is dissociated by GTP hydrolysis, which is catalyzed by Ran GTPase-activating protein (RanGAP) or Ran binding protein 1 (RanBP1). (C) The transport of large cargoes is thought to require the dilation of the central channel of the NPC (inset). (D) Inner nuclear membrane (INM) proteins are co-translationally integrated into the endoplasmic reticulum membrane, which is continuous with the outer nuclear membrane (ONM), and then imported to the INM. Similar to (B), the transport of INM proteins is also dependent on the Ran-cycle and karyopherins that likely travel through the central channel, while the cargo protein is anchored in the membrane and somehow pulled through. Substantial structural changes within the NPC would be necessary to facilitate this transport event.
Figure 2
Structural analysis of the heptameric Nup84 complex. Approximately 85% of the protein mass of the heptamer has been determined by x-ray crystallography. High-resolution crystal structures of the yeast nucleoporins Seh1•Nup85, Nup120, Sec13•Nup145C•Nup84, and the human nucleoporins Nup107 (homolog of Nup84) and Nup133 were fitted into a three-dimensional electron microscopy (EM) reconstruction. The crystallized N-terminal and C-terminal domains (NTD and CTD, respectively) are indicated. Two hinge regions are indicated at which the heptamer displayed marked flexibility. A 90°-rotated view is shown on the right, with the dimension of the heptamer indicated.
Figure 3
A schematic model of the NPC. The model includes four concentric cylinders composed of integral pore membrane proteins (POMs), coat nups, adaptor nups and channel nups. Natively unfolded FG-repeats of a number of nups make up the transport barrier in the central channel and are indicated by a transparent plug. Note that the surface topology of the pore membrane involves both convex and concave curvatures.
Figure 4
Flexibility and dynamics of NPC components. Spheres represent β-propeller domains, while cylinders represent α-helical solenoid domains (A) Crystal structures of the Seh1•Nup85 hetero-octamer (yellow and green, respectively) in three different conformational states revealed a rotation and a hinge motion. (B) Sec13•Nup145C (orange and blue, respectively) forms a hetero-octamer. (C) Sec13•Nup145C•Nup84 (colored as in panel B, Nup84 in yellow) forms a subcomplex that matches a hinge observed in one conformation of the heptameric Nup84 complex as determined by EM. A straight arrangement of this trimer would correspond to the second conformation of this subcomplex as revealed by EM. Notably, the homodimerization and heterodimerization surfaces of Nup145C are overlapping and therefore promiscuous. (D) Nup133 binds to Nup120 via a short N-terminal segment at the end of an unstructured region. Since the two proteins are localized at opposite ends of the Nup84 complex, this finding suggests a head-to-tail arrangement of the heptamers. Contraction and expansion of the unstructured region may allow for the flexible tethering of Nup120 and Nup133. (E) Four different states of Nup58/Nup45 tetramers have been crystallographically observed. The sliding of two Nup58/Nup45 dimers (purple) alters the overall dimensions of the tetramer. Nup58/Nup45 refers to two alternatively spliced variants (Nup58 and Nup45) and the determined structure is common to both proteins.
Figure 5
The architecture of the COPII coat. The vesicle-coating COPII complex consists of only five proteins. Sec13 and Sec31 assemble the outer shell, while Sec23, Sec24 and the membrane bound Sar1•GTP assemble the inner shell of COPII. In contrast to the pore membrane domain, the curvature of membrane-bound vesicles is convex in all directions.
Figure 6
The COPII coat is dynamic. (A) The Sec13•Sec31 hetero-octamer (red) is the repeating unit of the outer shell of the COPII complex. A cuboctahedral arrangement made of 12 modules is depicted, but other larger geometries of COPII cages were also observed by EM. Sec13 and Sec31 are shown in orange and grey, respectively. The inner shell of the COPII complex has been omitted for clarity. (B) With increasing cage size, the decreasing curvature of the vesicle is accounted for by a hinge motion that leads to more extended conformations of the Sec13•Sec31 hetero-octamer. Spheres represent β-propeller domains, while cylinders represent α-helical solenoid domains. Although Sec13 interacts with Sec31 and Nup145C in an analogous fashion, the higher-order organization differs and is facilitated by an additional β-propeller domain in Sec31 that is absent in Nup145C. The Sec13 binding site in Sec31 is located between the N-terminal β-propeller domain and the C-terminal α-helical solenoid domain.
Similar articles
- Structural evidence for common ancestry of the nuclear pore complex and vesicle coats.
Brohawn SG, Leksa NC, Spear ED, Rajashankar KR, Schwartz TU. Brohawn SG, et al. Science. 2008 Nov 28;322(5906):1369-73. doi: 10.1126/science.1165886. Epub 2008 Oct 30. Science. 2008. PMID: 18974315 Free PMC article. - Molecular architecture of the Nup84-Nup145C-Sec13 edge element in the nuclear pore complex lattice.
Brohawn SG, Schwartz TU. Brohawn SG, et al. Nat Struct Mol Biol. 2009 Nov;16(11):1173-7. doi: 10.1038/nsmb.1713. Epub 2009 Oct 25. Nat Struct Mol Biol. 2009. PMID: 19855394 Free PMC article. - The structure of the nuclear pore complex.
Hoelz A, Debler EW, Blobel G. Hoelz A, et al. Annu Rev Biochem. 2011;80:613-43. doi: 10.1146/annurev-biochem-060109-151030. Annu Rev Biochem. 2011. PMID: 21495847 Review. - Integrative structure-function mapping of the nucleoporin Nup133 suggests a conserved mechanism for membrane anchoring of the nuclear pore complex.
Kim SJ, Fernandez-Martinez J, Sampathkumar P, Martel A, Matsui T, Tsuruta H, Weiss TM, Shi Y, Markina-Inarrairaegui A, Bonanno JB, Sauder JM, Burley SK, Chait BT, Almo SC, Rout MP, Sali A. Kim SJ, et al. Mol Cell Proteomics. 2014 Nov;13(11):2911-26. doi: 10.1074/mcp.M114.040915. Epub 2014 Aug 19. Mol Cell Proteomics. 2014. PMID: 25139911 Free PMC article. - Membrane-coating lattice scaffolds in the nuclear pore and vesicle coats: commonalities, differences, challenges.
Leksa NC, Schwartz TU. Leksa NC, et al. Nucleus. 2010 Jul-Aug;1(4):314-8. doi: 10.4161/nucl.1.4.11798. Epub 2010 Mar 12. Nucleus. 2010. PMID: 21327078 Free PMC article. Review.
Cited by
- Crystal structure of the N-terminal domain of Nup358/RanBP2.
Kassube SA, Stuwe T, Lin DH, Antonuk CD, Napetschnig J, Blobel G, Hoelz A. Kassube SA, et al. J Mol Biol. 2012 Nov 9;423(5):752-65. doi: 10.1016/j.jmb.2012.08.026. Epub 2012 Sep 7. J Mol Biol. 2012. PMID: 22959972 Free PMC article. - Nuclear pores. Architecture of the nuclear pore complex coat.
Stuwe T, Correia AR, Lin DH, Paduch M, Lu VT, Kossiakoff AA, Hoelz A. Stuwe T, et al. Science. 2015 Mar 6;347(6226):1148-52. doi: 10.1126/science.aaa4136. Epub 2015 Feb 12. Science. 2015. PMID: 25745173 Free PMC article. - The Structure of the Nuclear Pore Complex (An Update).
Lin DH, Hoelz A. Lin DH, et al. Annu Rev Biochem. 2019 Jun 20;88:725-783. doi: 10.1146/annurev-biochem-062917-011901. Epub 2019 Mar 18. Annu Rev Biochem. 2019. PMID: 30883195 Free PMC article. Review. - Structure of the pre-mRNA leakage 39-kDa protein reveals a single domain of integrated zf-C3HC and Rsm1 modules.
Hashimoto H, Ramirez DH, Lautier O, Pawlak N, Blobel G, Palancade B, Debler EW. Hashimoto H, et al. Sci Rep. 2022 Oct 21;12(1):17691. doi: 10.1038/s41598-022-22183-3. Sci Rep. 2022. PMID: 36271106 Free PMC article. - Evidence for an evolutionary relationship between the large adaptor nucleoporin Nup192 and karyopherins.
Stuwe T, Lin DH, Collins LN, Hurt E, Hoelz A. Stuwe T, et al. Proc Natl Acad Sci U S A. 2014 Feb 18;111(7):2530-5. doi: 10.1073/pnas.1311081111. Epub 2014 Feb 6. Proc Natl Acad Sci U S A. 2014. PMID: 24505056 Free PMC article.
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of the cell. New York: Garland Science. 2008
- Green NM. The nuclear pore—a biological grommet? Nature. 1982;297:287–288. - PubMed
- Suntharalingam M, Wente SR. Peering through the pore: nuclear pore complex structure, assembly and function. Dev Cell. 2003;4:775–789. - PubMed
- Vlcek S, Foisner R. Lamins and lamin-associated proteins in aging and disease. Curr Opin Cell Biol. 2007;19:298–304. - PubMed
- Burke B, Stewart CL. The laminopathies: the functional architecture of the nucleus and its contribution to disease. Annu Rev Genomics Hum Genet. 2006;7:369–405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous