Immune responses to gut microbiota-commensals and pathogens - PubMed (original) (raw)
Immune responses to gut microbiota-commensals and pathogens
Takeshi Tanoue et al. Gut Microbes. 2010 Jul.
Abstract
The mammalian alimentary tract harbors hundreds of species of commensal microorganisms that intimately interact with the host immune system. Within the gut, the immune system actively reacts with potentially pathogenic microbes, while simultaneously remaining ignorant towards the vast majority of non-pathogenic microbiota. The disruption of this delicate balance results in inflammatory bowel diseases. In this review, we describe the recent advances in our understanding of how host-microbiota interactions shape the immune system and how they affect the responses against pathogenic bacteria.
Figures
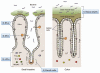
Figure 1
Multiple layers of the barrier system in the gut. (1) Mucus is secreted by goblet cells and forms two layers above the epithelial cells. In the colon, although the outer layer is loose, the inner layer is densely packed, firmly attached to the epithelium, and devoid of bacteria. In the small intestine, the mucus directly forms a soluble gel and is not attached to the epithelial cells. (2) Paneth cells are secretory epithelial cells located at the ends of the crypts within the small intestine. They secrete antimicrobial peptides and lysozyme into the crypt lumen, thereby regulating the composition of commensals and eliminating pathogens. (3) Intraepithelial lymphocytes (IELs) represent a unique population of mostly CD8+ T lymphocytes that reside within the epithelial cell layer of the intestinal mucosa. IELs have the capacity to kill infected and distressed intestinal epithelial cells in a non-antigen specific manner to avoid the spread of pathogens. (4) A subset of lamina propria (LP) dendritic cells (DCs) actively sample luminal contents through the formation of transepithelial dendrites and transmit signals to LP lymphocytes. (5) The LP is a thin layer of loose connective tissue which lies beneath the epithelium. The LP contains large numbers of effector, as well as regulatory, lymphocytes (LPLs) even in the absence of disease.
Figure 2
Segmented filamentous bacteria (SFB). Scanning electron micrographs of the Peyer's patch area of the ileum in mice colonized with SFB. The left image shows SFB distributed on both the follicle-associated epithelium (FAE) of the Peyer's patch and the absorptive epithelium of the villi in the ileum. The region indicated by the white square is shown at higher magnification in the right part. SFB are characterized by their long filamentous shape, with defined septa between each segment, and their attachment to epithelial cells.
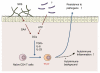
Figure 3
Model for segmented filamentous bacteria (SFB)-mediated induction of Th17 cells. Upon colonization of SFB in the small intestine, the epithelial cells release SAA. SAA is recognized by LP DCs that, in-turn, release IL-6 and IL-23, resulting in the activation of naïve CD4 T+ cells to become Th17 cells. SFB colonization and subsequent Th17 differentiation result in enhanced resistance to intestinal pathogens such as Citrobacter rodentium. However, in genetically autoimmune-prone individuals, this SFB-mediated Th17 induction may also enhance autoimmune inflammation. In addition to SFB, there may be other Th17-inducing bacterial species that produce extracellular ATP, which also activates LP DCs.
Similar articles
- The Roles of Inflammation, Nutrient Availability and the Commensal Microbiota in Enteric Pathogen Infection.
Stecher B. Stecher B. Microbiol Spectr. 2015 Jun;3(3). doi: 10.1128/microbiolspec.MBP-0008-2014. Microbiol Spectr. 2015. PMID: 26185088 - Bioderived materials that disarm the gut mucosal immune system: Potential lessons from commensal microbiota.
Harriman R, Lewis JS. Harriman R, et al. Acta Biomater. 2021 Oct 1;133:187-207. doi: 10.1016/j.actbio.2021.05.045. Epub 2021 Jun 5. Acta Biomater. 2021. PMID: 34098091 Review. - Regulation of innate and adaptive immunity by the commensal microbiota.
Jarchum I, Pamer EG. Jarchum I, et al. Curr Opin Immunol. 2011 Jun;23(3):353-60. doi: 10.1016/j.coi.2011.03.001. Epub 2011 Apr 3. Curr Opin Immunol. 2011. PMID: 21466955 Free PMC article. Review. - Lipopolysaccharide structures of Gram-negative populations in the gut microbiota and effects on host interactions.
Di Lorenzo F, De Castro C, Silipo A, Molinaro A. Di Lorenzo F, et al. FEMS Microbiol Rev. 2019 May 1;43(3):257-272. doi: 10.1093/femsre/fuz002. FEMS Microbiol Rev. 2019. PMID: 30649292 Review. - Role of intestinal microbiota and metabolites on gut homeostasis and human diseases.
Lin L, Zhang J. Lin L, et al. BMC Immunol. 2017 Jan 6;18(1):2. doi: 10.1186/s12865-016-0187-3. BMC Immunol. 2017. PMID: 28061847 Free PMC article. Review.
Cited by
- Intestinal permeability disturbances: causes, diseases and therapy.
Macura B, Kiecka A, Szczepanik M. Macura B, et al. Clin Exp Med. 2024 Sep 28;24(1):232. doi: 10.1007/s10238-024-01496-9. Clin Exp Med. 2024. PMID: 39340718 Free PMC article. Review. - Gut protozoa of wild rodents - a meta-analysis.
Hunter-Barnett S, Viney M. Hunter-Barnett S, et al. Parasitology. 2024 May;151(6):594-605. doi: 10.1017/S0031182024000556. Epub 2024 May 8. Parasitology. 2024. PMID: 38714350 Free PMC article. - A Comprehensive Exploration of Therapeutic Strategies in Inflammatory Bowel Diseases: Insights from Human and Animal Studies.
Dias IE, Dias IR, Franchi-Mendes T, Viegas CA, Carvalho PP. Dias IE, et al. Biomedicines. 2024 Mar 26;12(4):735. doi: 10.3390/biomedicines12040735. Biomedicines. 2024. PMID: 38672091 Free PMC article. Review. - Antibiotic prescribing patterns and carriage of antibiotic-resistant Escherichia coli and Enterococcus species in healthy individuals from selected communities in Lusaka and Ndola districts, Zambia.
Yamba K, Mudenda S, Mpabalwani E, Mainda G, Mukuma M, Samutela MT, Lukwesa C, Chizimu J, Kaluba CK, Mutalange M, Chilengi R, Muma JB. Yamba K, et al. JAC Antimicrob Resist. 2024 Mar 5;6(2):dlae027. doi: 10.1093/jacamr/dlae027. eCollection 2024 Apr. JAC Antimicrob Resist. 2024. PMID: 38449515 Free PMC article. - The Role of the Microbiome in the Pathogenesis and Treatment of Ulcerative Colitis-A Literature Review.
Świrkosz G, Szczygieł A, Logoń K, Wrześniewska M, Gomułka K. Świrkosz G, et al. Biomedicines. 2023 Nov 25;11(12):3144. doi: 10.3390/biomedicines11123144. Biomedicines. 2023. PMID: 38137365 Free PMC article. Review.
References
- Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources