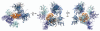Membrane-coating lattice scaffolds in the nuclear pore and vesicle coats: commonalities, differences, challenges - PubMed (original) (raw)
Review
Membrane-coating lattice scaffolds in the nuclear pore and vesicle coats: commonalities, differences, challenges
Nina C Leksa et al. Nucleus. 2010 Jul-Aug.
Abstract
The nuclear pore complex (NPC) regulates all traffic between the cytoplasm and the nucleus. It is a large protein assembly composed of multiple copies of ∼30 nucleoporins (nups). Structural studies of the NPC have been limited by its considerable size and complexity. Progress toward understanding the structure of this nanomachine has benefited from its modular nature, which allows for this 40-60 MDa assembly to be broken down into subcomplexes that can be studied individually. While recent work by both crystallographers and electron microscopists has greatly enhanced our model of the NPC, the resolution gap between crystal and EM structures remains too large to confidently place individual proteins within the context of the fully assembled NPC. In an effort to arrive at a veritable model of the NPC, we solved the structure of several scaffold nups and defined the ancestral coatomer element (ACE1) common to a set of nucleoporins and COPII vesicle coat proteins. Subsequently, we proposed a lattice-like model of the NPC, analogous to the COPII lattice, in which ACE1 proteins form the edge elements and β-propellers form the vertex elements. Here, we review our recent studies, speculate on how interactions between subcomplexes of the NPC are mediated, and outline the steps and challenges that lay ahead on the path to understanding this enormous assembly in molecular detail.
Keywords: ACE1; assembly; membrane-coating; nuclear pore complex; nucleoporin; ²-propeller.
Figures
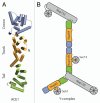
Figure 1
Schematic of ACE1 and the Y-complex. (A) The three modules of the ACE1 domain—crown, trunk and tail—are shown in blue, orange and green, respectively. The positions of the N and C termini are indicated. (B) The relative positions of the nups within the Y complex are shown. ACE1 nups are colored as in (A), all others in gray.
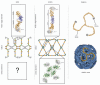
Figure 2
Membrane-coating scaffolds of the NPC and vesicle coats. Schematics of the lattice like assemblies of the NPC and COPII scaffolds are shown, with ACE1 modules colored as in Figure 1. Enlarged views of the ACE1 edge elements are shown for both the NPC and COPII, in addition to the vertex element of the COPII lattice (with Sec31 shown in green and Sec13 shown in gray). The assembly of the vertex element, of the NPC, including which ²-propellers are used, is currently unknown. While the COPII ACE1 interaction is homotypic, the analogous ACE1 interaction between Nup145C and Nup84 is heterotypic. A clathrin triskelion and the entire clathrin coat are shown in the right panel. While the clathrin coat is similarly composed of helical and ²-propeller domains (colored in orange and gray, respectively), overall structure and assembly differ significantly from the NPC and COPII lattices.
Figure 3
Crystal contacts of the Sec13 ²-propeller. A Sec13 reference molecule is shown in orange, with neighboring molecules from five different crystal structures (PDB accession codes 2PM6, 2PM9, 3BG1, 3JRO, 3JRP) superimposed to indicate the number and variety of ²-propeller-²-propeller contacts that the Sec13 molecule can make in the crystal. Neighboring molecules from the same crystal are colored in the same shade of blue.
Similar articles
- Molecular architecture of the Nup84-Nup145C-Sec13 edge element in the nuclear pore complex lattice.
Brohawn SG, Schwartz TU. Brohawn SG, et al. Nat Struct Mol Biol. 2009 Nov;16(11):1173-7. doi: 10.1038/nsmb.1713. Epub 2009 Oct 25. Nat Struct Mol Biol. 2009. PMID: 19855394 Free PMC article. - Structural evidence for common ancestry of the nuclear pore complex and vesicle coats.
Brohawn SG, Leksa NC, Spear ED, Rajashankar KR, Schwartz TU. Brohawn SG, et al. Science. 2008 Nov 28;322(5906):1369-73. doi: 10.1126/science.1165886. Epub 2008 Oct 30. Science. 2008. PMID: 18974315 Free PMC article. - Structure of the Sec13-Sec16 edge element, a template for assembly of the COPII vesicle coat.
Whittle JR, Schwartz TU. Whittle JR, et al. J Cell Biol. 2010 Aug 9;190(3):347-61. doi: 10.1083/jcb.201003092. J Cell Biol. 2010. PMID: 20696705 Free PMC article. - From the resolution revolution to evolution: structural insights into the evolutionary relationships between vesicle coats and the nuclear pore.
Beck M, Mosalaganti S, Kosinski J. Beck M, et al. Curr Opin Struct Biol. 2018 Oct;52:32-40. doi: 10.1016/j.sbi.2018.07.012. Epub 2018 Aug 11. Curr Opin Struct Biol. 2018. PMID: 30103204 Review. - Structure and Assembly of the Nuclear Pore Complex.
Hampoelz B, Andres-Pons A, Kastritis P, Beck M. Hampoelz B, et al. Annu Rev Biophys. 2019 May 6;48:515-536. doi: 10.1146/annurev-biophys-052118-115308. Epub 2019 Apr 3. Annu Rev Biophys. 2019. PMID: 30943044 Review.
Cited by
- A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1.
Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. Bar-Peled L, et al. Science. 2013 May 31;340(6136):1100-6. doi: 10.1126/science.1232044. Science. 2013. PMID: 23723238 Free PMC article. - Integrative structure-function mapping of the nucleoporin Nup133 suggests a conserved mechanism for membrane anchoring of the nuclear pore complex.
Kim SJ, Fernandez-Martinez J, Sampathkumar P, Martel A, Matsui T, Tsuruta H, Weiss TM, Shi Y, Markina-Inarrairaegui A, Bonanno JB, Sauder JM, Burley SK, Chait BT, Almo SC, Rout MP, Sali A. Kim SJ, et al. Mol Cell Proteomics. 2014 Nov;13(11):2911-26. doi: 10.1074/mcp.M114.040915. Epub 2014 Aug 19. Mol Cell Proteomics. 2014. PMID: 25139911 Free PMC article. - The nuclear pore complex function of Sec13 protein is required for cell survival during retinal development.
Niu X, Hong J, Zheng X, Melville DB, Knapik EW, Meng A, Peng J. Niu X, et al. J Biol Chem. 2014 Apr 25;289(17):11971-11985. doi: 10.1074/jbc.M114.547190. Epub 2014 Mar 13. J Biol Chem. 2014. PMID: 24627485 Free PMC article. - GATOR2-dependent mTORC1 activity is a therapeutic vulnerability in FOXO1 fusion-positive rhabdomyosarcoma.
Morales J, Allegakoen DV, Garcia JA, Kwong K, Sahu PK, Fajardo DA, Pan Y, Horlbeck MA, Weissman JS, Gustafson WC, Bivona TG, Sabnis AJ. Morales J, et al. JCI Insight. 2022 Dec 8;7(23):e162207. doi: 10.1172/jci.insight.162207. JCI Insight. 2022. PMID: 36282590 Free PMC article. - Evolution of a transcriptional regulator from a transmembrane nucleoporin.
Franks TM, Benner C, Narvaiza I, Marchetto MC, Young JM, Malik HS, Gage FH, Hetzer MW. Franks TM, et al. Genes Dev. 2016 May 15;30(10):1155-71. doi: 10.1101/gad.280941.116. Epub 2016 May 19. Genes Dev. 2016. PMID: 27198230 Free PMC article.
References
- Tran EJ, Wente SR. Dynamic nuclear pore complexes: life on the edge. Cell. 2006;125:1041–1053. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous