Assessment of myocardial fibrosis with cardiovascular magnetic resonance - PubMed (original) (raw)
Review
Assessment of myocardial fibrosis with cardiovascular magnetic resonance
Nathan Mewton et al. J Am Coll Cardiol. 2011.
Abstract
Diffuse interstitial or replacement myocardial fibrosis is a common feature of a broad variety of cardiomyopathies. Myocardial fibrosis leads to impaired cardiac diastolic and systolic function and is related to adverse cardiovascular events. Cardiovascular magnetic resonance (CMR) may uniquely characterize the extent of replacement fibrosis and may have prognostic value in various cardiomyopathies. Myocardial longitudinal relaxation time mapping is an emerging technique that could improve CMR's diagnostic accuracy, especially for interstitial diffuse myocardial fibrosis. As such, CMR could be integrated in the monitoring and therapeutic management of a large number of patients. This review summarizes the advantages and limitations of CMR for the assessment of myocardial fibrosis.
Copyright © 2011 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
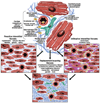
Etiophysiopathology of Myocardial Fibrosis. Myocardial fibrosis is a complex process that involves each cellular component of the myocardial tissue. The myocardial fibroblast has a central position in this process by increasing the production of collagen and other extracellular matrix components under the influence of various factors (renin-angiotensin system, myocyte apoptosis, pro-inflammatory cytokines, reactive oxygen species).
Figure 2
Diabetic cardiomyopathy: mild myocardial interstitial fibrosis stained in blue with Masson trichrome (white arrow) in a patient with long-duration type 1 diabetes mellitus at autopsy, with perivascular fibrosis (A) and mild fibrosis between myocytes (B). Reprinted with permission Konduracka et al. (80)
Figure 3
A) T1 map Construction. T1 map after 15 minutes of gadolinium administration in a inferior infarct case. This is the modified look-locker inversion recovery sequence that uses 17 heart-beats to reconstruct 11 images with different inversion times during mid-diastole. It is necessary to combine all images to generate the final T1 map. For that, it is necessary to apply algorithms to define the best fitting curve over the 11 acquired initial voxels linking for the same location. Those fitting algorithm are very sensitive to motion and image quality/artifacts. The result is a T1 map imaging where the T1 time for the global or segmented LV can be assessed. B) T1 Recovery Graph after Contrast Administration. Graph showing the recovery of absolute myocardial T1 value in a healthy heart (short-axis, mid-ventricle) at different time points prior and after contrast administration (0, 2, 4, 6, 8, 10, 15 and 20 minutes). T1 values are expressed as means ± standard deviations. The global and regional mean T1 values will vary significantly significantly with the time of assessment. The standard deviation of T1 value is more significant prior to contrast administration. Reprinted with permission, from Messroghli et al. (70)
Figure 3
A) T1 map Construction. T1 map after 15 minutes of gadolinium administration in a inferior infarct case. This is the modified look-locker inversion recovery sequence that uses 17 heart-beats to reconstruct 11 images with different inversion times during mid-diastole. It is necessary to combine all images to generate the final T1 map. For that, it is necessary to apply algorithms to define the best fitting curve over the 11 acquired initial voxels linking for the same location. Those fitting algorithm are very sensitive to motion and image quality/artifacts. The result is a T1 map imaging where the T1 time for the global or segmented LV can be assessed. B) T1 Recovery Graph after Contrast Administration. Graph showing the recovery of absolute myocardial T1 value in a healthy heart (short-axis, mid-ventricle) at different time points prior and after contrast administration (0, 2, 4, 6, 8, 10, 15 and 20 minutes). T1 values are expressed as means ± standard deviations. The global and regional mean T1 values will vary significantly significantly with the time of assessment. The standard deviation of T1 value is more significant prior to contrast administration. Reprinted with permission, from Messroghli et al. (70)
Figure 4
T1 maps of the myocardium at the mid-ventricular short axis level in a healthy volunteer, a) Pre-contrast, b) post gadolinium contrast (0.15 mmol/kg) at 12 minutes c) and 25 minutes acquired with the MOLLI sequence. The mean T1 value for the left ventricle (LV) can be obtained at each time after tracing the endocardial and epicardial countours of the LV (a).
Figure 5
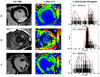
Comparison of late gadolinium enhanced studies with corresponding T1 maps and T1 values distribution histograms in different cardiomyopathies: A) Chronic inferior myocardial infarction; B) Cardiac amyloïdosis; C) Non Ischemic Dilated Cardiomyopathy. In each example, the short axis late gadolinium images shows images with different patterns of enhancement, transmural localized in the case of a myocardial infarction scar (A1), sub-endocardial diffuse in the case of cardiac amyloïdosis (B1) or sub-epicardial and heterogeneous in the case of dilated cardiomyopathy. In the middle panel are the corresponding T1 maps (A2, B2, B3) obtained after MOLLI acquisitions. From those T1 maps, a mean left ventricular (LV) T1 value can be obtained. This information can also be processed more precisely through the analysis of the distribution histogram of the LV T1 values. Very distinct patterns of distributions can be seen on those examples, but this has to be shown in further larger clinical studies. This might also be a new way to assess and quantify myocardial fibrosis.
Similar articles
- Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis--a clinical study using myocardial T1-mapping and extracellular volume quantification.
Ntusi NA, Piechnik SK, Francis JM, Ferreira VM, Rai AB, Matthews PM, Robson MD, Moon J, Wordsworth PB, Neubauer S, Karamitsos TD. Ntusi NA, et al. J Cardiovasc Magn Reson. 2014 Mar 4;16(1):21. doi: 10.1186/1532-429X-16-21. J Cardiovasc Magn Reson. 2014. PMID: 24593856 Free PMC article. - Cardiovascular magnetic resonance imaging to assess myocardial fibrosis in valvular heart disease.
Podlesnikar T, Delgado V, Bax JJ. Podlesnikar T, et al. Int J Cardiovasc Imaging. 2018 Jan;34(1):97-112. doi: 10.1007/s10554-017-1195-y. Epub 2017 Jun 22. Int J Cardiovasc Imaging. 2018. PMID: 28642994 Free PMC article. Review. - Histological Validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker imaging (MOLLI) T1 mapping at 3 T.
de Meester de Ravenstein C, Bouzin C, Lazam S, Boulif J, Amzulescu M, Melchior J, Pasquet A, Vancraeynest D, Pouleur AC, Vanoverschelde JL, Gerber BL. de Meester de Ravenstein C, et al. J Cardiovasc Magn Reson. 2015 Jun 11;17(1):48. doi: 10.1186/s12968-015-0150-0. J Cardiovasc Magn Reson. 2015. PMID: 26062931 Free PMC article. - MR imaging assessment of myocardial edema with T2 mapping.
Montant P, Sigovan M, Revel D, Douek P. Montant P, et al. Diagn Interv Imaging. 2015 Sep;96(9):885-90. doi: 10.1016/j.diii.2014.07.008. Epub 2015 Feb 16. Diagn Interv Imaging. 2015. PMID: 25697831 Review. - Association between diffuse myocardial fibrosis by cardiac magnetic resonance contrast-enhanced T₁ mapping and subclinical myocardial dysfunction in diabetic patients: a pilot study.
Ng AC, Auger D, Delgado V, van Elderen SG, Bertini M, Siebelink HM, van der Geest RJ, Bonetti C, van der Velde ET, de Roos A, Smit JW, Leung DY, Bax JJ, Lamb HJ. Ng AC, et al. Circ Cardiovasc Imaging. 2012 Jan;5(1):51-9. doi: 10.1161/CIRCIMAGING.111.965608. Epub 2011 Dec 1. Circ Cardiovasc Imaging. 2012. PMID: 22135399
Cited by
- Myocardial involvement in end-stage renal disease patients with anemia as assessed by cardiovascular magnetic resonance native T1 mapping: An observational study.
Chen L, Xu R, Xu H, Yang Z, Zhang Y, Li Z, Xia C, Rao L, Guo Y. Chen L, et al. Medicine (Baltimore). 2024 Nov 15;103(46):e39724. doi: 10.1097/MD.0000000000039724. Medicine (Baltimore). 2024. PMID: 39560547 Free PMC article. - Quantification of Replacement Fibrosis in Aortic Stenosis: A Narrative Review on the Utility of Cardiovascular Magnetic Resonance Imaging.
Rajah MR, Doubell AF, Herbst PG. Rajah MR, et al. Diagnostics (Basel). 2024 Oct 31;14(21):2435. doi: 10.3390/diagnostics14212435. Diagnostics (Basel). 2024. PMID: 39518402 Free PMC article. Review. - Improvement of Quantification of Myocardial Synthetic ECV with Second-Generation Deep Learning Reconstruction.
Morioka T, Kato S, Onoma A, Izumi T, Sakano T, Ishikawa E, Sawamura S, Yasuda N, Nagase H, Utsunomiya D. Morioka T, et al. J Cardiovasc Dev Dis. 2024 Oct 2;11(10):304. doi: 10.3390/jcdd11100304. J Cardiovasc Dev Dis. 2024. PMID: 39452275 Free PMC article. - Incremental Prognostic Value of Cardiac MRI Feature Tracking and T1 Mapping in Arrhythmogenic Right Ventricular Cardiomyopathy.
Lu G, Cao L, Ye W, Wei X, Xie J, Du Z, Zhang X, Luo X, Ou J, Zhang Q, Liu Y, Yang Y, Liu H. Lu G, et al. Radiol Cardiothorac Imaging. 2024 Oct;6(5):e230430. doi: 10.1148/ryct.230430. Radiol Cardiothorac Imaging. 2024. PMID: 39446042 Free PMC article. - Extracellular Volume and Fibrosis Volume of Left Ventricular Myocardium Assessed by Cardiac Magnetic Resonance in Vaccinated and Unvaccinated Patients with a History of SARS-CoV-2 Infection.
Gać P, Hajdusianek W, Żórawik A, Poręba M, Poręba R. Gać P, et al. Cardiovasc Toxicol. 2024 Dec;24(12):1455-1466. doi: 10.1007/s12012-024-09929-3. Epub 2024 Oct 15. Cardiovasc Toxicol. 2024. PMID: 39404974 Free PMC article.
References
- de Leeuw N, Ruiter DJ, Balk AH, de Jonge N, Melchers WJ, Galama JM. Histopathologic findings in explanted heart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int. 2001;14:299–306. - PubMed
- Marijianowski MM, Teeling P, Mann J, Becker AE. Dilated cardiomyopathy is associated with an increase in the type I/type III collagen ratio: a quantitative assessment. J Am Coll Cardiol. 1995;25:1263–1272. - PubMed
- Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517. - PubMed
- Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OH. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation. 1995;91:161–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 HL101397/HL/NHLBI NIH HHS/United States
- P20 HL101397-01/HL/NHLBI NIH HHS/United States
- P20 HL101397-01S1/HL/NHLBI NIH HHS/United States
- P20 HL101397-02/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials