CP47,497-C8 and JWH073, commonly found in 'Spice' herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists - PubMed (original) (raw)
CP47,497-C8 and JWH073, commonly found in 'Spice' herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists
Brady K Atwood et al. Eur J Pharmacol. 2011.
Abstract
'Spice' is an herbal blend that has been reported to produce cannabis-like effects when smoked and is marketed as an alternative to marijuana. Synthetic additives have been identified in numerous 'Spice' preparations from different sources. Common among many of the preparations were the compounds JWH018 and a dimethyloctyl variant of CP47,497 (CP47,497-C8) and, more recently JWH073. The synaptic effects of each of these compounds were uncharacterized. We previously reported that JWH018 is a potent and efficacious CB(1) cannabinoid receptor agonist. In this study we have examined the abilities of CP47,497-C8 and JWH073 to inhibit neurotransmission in cultured autaptic hippocampal neurons. Each inhibited EPSCs with an efficacy and potency similar to JWH018. We also analyzed these compounds' effects on promoting internalization of CB(1) receptors in HEK293 cells stably expressing CB(1) receptors. Similar to our neurotransmission data, CP47,497-C8 internalized CB(1) in a fashion indistinguishable from JWH018. However, JWH073 was less potent and produced slower internalization than JWH018 and CP47,497-C8. It appears that 'Spice' contains a number of cannabinoid receptor agonists that activate CB(1) receptors to inhibit synaptic transmission with similar potencies and efficacies. It is highly probable that the cannabis-like effects of 'Spice' are due to the presence of these and analogous synthetic additives acting on CB(1) receptors.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
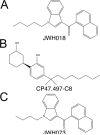
Figure 1. The chemical structures of (A) JWH018, (B) CP47,497-C8, (C) JWH073
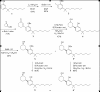
Figure 2. Scheme for the synthesis of cis- and trans-CP47,497-C8
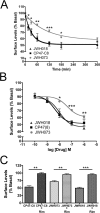
Figure 3. CP47,497-C8 and JWH073 decreases neurotransmitter release by activating CB1receptors
(A) A representative experiment showing a time course of EPSC inhibition by CP47,497-C8 with the indicated concentrations. 1 μM rimonabant reverses CP47,497-C8 inhibition. Inset shows representative traces for three indicated time points. (B) A representative experiment showing a time course of EPSC inhibition by JWH073 with the indicated concentrations. 1 μM rimonabant, a CB1 receptor antagonist, reverses JWH073 inhibition. Inset shows representative traces for three indicated time points. (C) Concentration dependence of CP47,497-C8- and JWH073-induced suppression of neurotransmission (n= 5 to 8 for each concentration tested). (D) Quantification of 1 μM rimonabant inhibitory effect on 1 μM drug treatment (n = 7 to 8 for each concentration) Values are presented as mean ± S.E.M. where applicable. Data analyzed using unpaired Student's t-tests. ***P < 0.0001.
Figure 4. Prolonged treatment with CP47,497-C8 or JWH073 promotes CB1 receptor internalization
(A) In CB1 expressing HEK293 cells 100nM CP47,497-C8, JWH073 or JWH018 treatment results in robust receptor internalization (3 replicate samples from 5-14 independent experiments, except for 360 min time point: 3–4 experiments). (B) Following 2 hours of exposure to increasing concentrations of each drug, CB1 internalized in a concentration-dependent manner (3 replicate samples from 4 to 14 independent experiments). (C) 1 μM rimonabant prevents receptor internalization by each drug treatment. Values are presented as mean ± S.E.M. Data in (A) and (B) analyzed using one-way ANOVA with Bonferroni's multiple comparison test. Data in (C) analyzed using unpaired Student's t-test. *P < 0.05, ** P < 0.01, *** P < 0.0001.
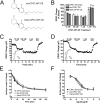
Figure 5. Individual stereoisomers of CP47,497-C8 do not differ from the stereoisomeric mixture in their effects on neurotransmission and CB1 receptor internalization
(A) Chemical structures of cis-CP47,497-C8 and trans-CP-47,497-C8. (B) Summary of the amount of EPSC suppression achieved by indicated concentrations of each stereostereoisomer. Data from Fig. 3C included for ease of comparison. 1 μM rimonabant treatment reverses the effects of treatments with 1 μM of each drug. **: P < 0.01 vs. 1 μM drug treatments (unpaired Student's t-tests). (C) A representative experiment showing a time course of EPSC inhibition by cis-CP47,497-C8 with the indicated concentrations. 1 μM rimonabant reverses inhibition. Inset shows representative traces for three indicated time points. (D) A representative experiment showing a time course of EPSC inhibition by trans-CP47,497-C8 with the indicated concentrations. 1 μM rimonabant reverses inhibition. Inset shows representative traces for three indicated time points. (E) In CB1 expressing HEK293 cells 100nM cis-CP47,497-C8 or trans-CP47,497-C8 treatment results in robust receptor internalization (3 replicate samples from 5–10 independent experiments. Data from Fig. 4A have been included for ease of comparison (F) Following 2 hours of exposure to increasing concentrations of each drug, CB1 was internalized in a concentration-dependent manner (3 replicate samples from 5–10 independent experiments. Values are presented as mean ± S.E.M where appropriate. Data analyzed using one-way ANOVA with Bonferroni's multiple comparison tests (except where indicated in (B)).
Similar articles
- JWH018, a common constituent of 'Spice' herbal blends, is a potent and efficacious cannabinoid CB receptor agonist.
Atwood BK, Huffman J, Straiker A, Mackie K. Atwood BK, et al. Br J Pharmacol. 2010 Jun;160(3):585-93. doi: 10.1111/j.1476-5381.2009.00582.x. Epub 2010 Jan 22. Br J Pharmacol. 2010. PMID: 20100276 Free PMC article. - Disruption of hippocampal synaptic transmission and long-term potentiation by psychoactive synthetic cannabinoid 'Spice' compounds: comparison with Δ9 -tetrahydrocannabinol.
Hoffman AF, Lycas MD, Kaczmarzyk JR, Spivak CE, Baumann MH, Lupica CR. Hoffman AF, et al. Addict Biol. 2017 Mar;22(2):390-399. doi: 10.1111/adb.12334. Epub 2016 Jan 5. Addict Biol. 2017. PMID: 26732435 Free PMC article. - CB1 Receptor Negative Allosteric Modulators as a Potential Tool to Reverse Cannabinoid Toxicity.
Flavin A, Azizi P, Murataeva N, Yust K, Du W, Ross R, Greig I, Nguyen T, Zhang Y, Mackie K, Straiker A. Flavin A, et al. Molecules. 2024 Apr 20;29(8):1881. doi: 10.3390/molecules29081881. Molecules. 2024. PMID: 38675703 Free PMC article. - Tripping with Synthetic Cannabinoids ("Spice"): Anecdotal and Experimental Observations in Animals and Man.
Järbe TUC, Raghav JG. Järbe TUC, et al. Curr Top Behav Neurosci. 2017;32:263-281. doi: 10.1007/7854_2016_16. Curr Top Behav Neurosci. 2017. PMID: 27753006 Review. - [Drug discrimination properties and cytotoxicity of the cannabinoid receptor ligands].
Tomiyama K, Funada M. Tomiyama K, et al. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2012 Jun;47(3):135-43. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2012. PMID: 22894054 Review. Japanese.
Cited by
- Sphingosine lysolipids in the CNS: endogenous cannabinoid antagonists or a parallel pain modulatory system?
Selley DE, Welch SP, Sim-Selley LJ. Selley DE, et al. Life Sci. 2013 Aug 14;93(5-6):187-93. doi: 10.1016/j.lfs.2013.06.004. Epub 2013 Jun 16. Life Sci. 2013. PMID: 23782998 Free PMC article. Review. - Development of a high-performance liquid chromatography-tandem mass spectrometry method for the identification and quantification of CP-47,497, CP-47,497-C8 and JWH-250 in mouse brain.
Samano KL, Poklis JL, Lichtman AH, Poklis A. Samano KL, et al. J Anal Toxicol. 2014 Jul-Aug;38(6):307-14. doi: 10.1093/jat/bku043. Epub 2014 May 9. J Anal Toxicol. 2014. PMID: 24816398 Free PMC article. - Methodology for controlled administration of smoked synthetic cannabinoids JWH-018 and JWH-073.
Cooper ZD, Poklis JL, Liu F. Cooper ZD, et al. Neuropharmacology. 2018 May 15;134(Pt A):92-100. doi: 10.1016/j.neuropharm.2017.11.020. Epub 2017 Nov 14. Neuropharmacology. 2018. PMID: 29146503 Free PMC article. - Beyond THC: The New Generation of Cannabinoid Designer Drugs.
Fattore L, Fratta W. Fattore L, et al. Front Behav Neurosci. 2011 Sep 21;5:60. doi: 10.3389/fnbeh.2011.00060. eCollection 2011. Front Behav Neurosci. 2011. PMID: 22007163 Free PMC article. - Effects of acute and repeated dosing of the synthetic cannabinoid CP55,940 on intracranial self-stimulation in mice.
Grim TW, Wiebelhaus JM, Morales AJ, Negus SS, Lichtman AH. Grim TW, et al. Drug Alcohol Depend. 2015 May 1;150:31-7. doi: 10.1016/j.drugalcdep.2015.01.022. Epub 2015 Jan 29. Drug Alcohol Depend. 2015. PMID: 25772438 Free PMC article.
References
- Aung MM, Griffin G, Huffman JW, Wu M, Keel C, Yang B, Showalter VM, Abood ME, Martin BR. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor binding. Drug Alcohol Depend. 2000;60:133–140. - PubMed
- Auwarter V, Dresen S, Weinmann W, Muller M, Putz M, Ferreiros N. `Spice' and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom. 2009;44:832–837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA024122/DA/NIDA NIH HHS/United States
- UL1 RR025761/RR/NCRR NIH HHS/United States
- R01 DA011322-11A1/DA/NIDA NIH HHS/United States
- R21 DA024122/DA/NIDA NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- R01 DA011322/DA/NIDA NIH HHS/United States
- R21 DA024122-02/DA/NIDA NIH HHS/United States
- K05 DA021696-04/DA/NIDA NIH HHS/United States
- UL1 RR025761-03/RR/NCRR NIH HHS/United States
- DA009158/DA/NIDA NIH HHS/United States
- K05 DA021696/DA/NIDA NIH HHS/United States
- P01 DA009158/DA/NIDA NIH HHS/United States
- DA11322/DA/NIDA NIH HHS/United States
- DA21696/DA/NIDA NIH HHS/United States
- P01 DA009158-13/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources