Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer - PubMed (original) (raw)
Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer
Lucia De Monte et al. J Exp Med. 2011.
Abstract
Pancreatic cancer is a very aggressive disease characterized by a marked desmoplasia with a predominant Th2 (GATA-3+) over Th1 (T-bet+) lymphoid infiltrate. We found that the ratio of GATA-3+/T-bet+ tumor-infiltrating lymphoid cells is an independent predictive marker of patient survival. Patients surgically treated for stage IB/III disease with a ratio inferior to the median value had a statistically significant prolonged overall survival, implying an active role for Th2 responses in disease progression. Thymic stromal lymphopoietin (TSLP), which favors Th2 cell polarization through myeloid dendritic cell (DC) conditioning, was secreted by cancer-associated fibroblasts (CAFs) after activation with tumor-derived tumor necrosis factor α and interleukin 1β. TSLP-containing supernatants from activated CAFs induced in vitro myeloid DCs to up-regulate the TSLP receptor (TSLPR), secrete Th2-attracting chemokines, and acquire TSLP-dependent Th2-polarizing capability in vitro. In vivo, Th2 chemoattractants were expressed in the tumor and in the stroma, and TSLPR-expressing DCs were present in the tumor stroma and in tumor-draining but not in nondraining lymph nodes. Collectively, this study identifies in pancreatic cancer a cross talk between tumor cells and CAFs, resulting in a TSLP-dependent induction of Th2-type inflammation which associates with reduced patient survival. Thus, blocking TSLP production by CAFs might help to improve prognosis in pancreatic cancer.
Figures
Figure 1.
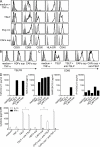
The ratio of G/T tumor-infiltrating lymphoid cells predicts survival after surgery in patients with stage IB/III pancreatic cancer. (A) Representative immunohistochemical analysis for lymphoid GATA-3 and T-bet staining in the tumor (n = 69; left) and normal pancreatic tissue from benign lesions (n = 3; right). The arrows indicate rare positive cells in normal tissue. Bars, 100 µm. (B) Percentage of GATA-3+ (circles) and T-bet+ (triangles) lymphoid cells for each of the analyzed tumor samples (n = 69). The values are significantly different and indicated as: ***, P < 0.001 (determined by paired one-tailed Student’s t test). (C) Waterfall plot of the G/T ratio for each tumor sample. The dashed line indicates the G/T ratio 5.2, which is the median value. (D) Kaplan-Meier curve for overall survival by median G/T ratio (5.2). Survival significantly decreased (P = 0.008) as a function of a G/T ratio ≥ 5.2.
Figure 2.
TSLP is expressed by CAFs, and its secretion is induced by proinflammatory cytokines released by tumor cells. (A) TSLP mRNA expression in tumor and surrounding tissues, tumor cell lines, CAFs, and HDFs. Each dot represents a different surgical sample or a different cell line. (B) TSLP is expressed in vivo in the stroma. LCM (left) was used to collect stromal (top) and epithelial (bottom) tumor cells from surgical specimens and TSLP mRNA expression analyzed (right; representative of tumor samples from three patients). The mRNA expression levels were normalized to the expression of GAPDH. TSLP mRNA expression of Caco2 cell line was used as calibrator, as in Rimoldi et al. (2005). (C) TSLP protein secretion by CAFs, HDFs, and tumor cell lines treated with proinflammatory cytokines as single agent or in combination. Left, 20 ng/ml TNF; middle, 10 ng/ml IL-1β; right, TNF plus IL-1β. Each dot represents a different cell line. (D) TNF (top) and IL-1β (bottom) mRNA expression in tumor and the corresponding surrounding tissue (each dot and corresponding triangle represent surgical samples from single patients). (E) TNF (left) and IL-1β (right) mRNA expression in tumor epithelial and stromal cells collected by LCM (top; representative of tumor samples from three patients) and in isolated and in vitro–cultured paired tumor cell lines and CAFs from the same patient (bottom; representative of tumor samples from three patients). Inset magnifies IL-1β expression in CAFs. (F) TSLP protein secretion by CAFs treated with supernatant from tumor cell lines (tumor cells sup) in the absence and presence of anti-TNF, anti–IL-1β, and isotype control Abs (representative of experiments performed with three different tumor cell lines and three CAFs). Data in A–F are means of at least duplicate determinations ± SD. Responses significantly different in A, C, D, and F are indicated as: *, P < 0.05; **, 0.001< P < 0.01 (determined by paired or unpaired one-tailed Student’s t test).
Figure 3.
Supernatant of TNF-treated CAF (CAF sup) activates in vitro myeloid DCs with features of TSLP-treated DCs. (A) FACS analysis of DCs after 24-h incubation with the indicated stimuli (representative of independent experiments, n = 3). Experiments were performed with supernatants from three different CAFs. Open histograms represent staining of DC activation markers; filled histograms represent the isotype control. (B) FACS analysis of TSLPR expression by DCs activated in the presence of medium plus TNF, supernatant of TNF-treated HDF (HDF sup; obtained from two different HDFs), and CAF sup (obtained from three different CAFs; representative of independent experiments n = 3). (C) FACS analysis of CD80 expression by DCs incubated with CAF sup (obtained from three different CAFs) in the absence and in the presence of an anti-TSLP Ab (representative of independent experiments, n = 4). Experiments with medium plus TNF and TSLP, in the absence and in the presence of the anti-TSLP Ab, are shown as negative and positive controls, respectively. (D) Chemokine production by DCs activated with the following stimuli: medium alone, 20 ng/ml TNF; 15 ng/ml TSLP; HDF sup and CAF sup (representative of three experiments performed with three different CAFs and two different HDFs). (E) CAF sup endows DCs with Th2 polarizing capability that depends on TSLP. CD4+CD45RA+ naive T cells were cultured with DCs previously activated with the indicated stimuli. At day 6, IFN-γ and IL-13 secreted by CD4+ T cells were tested by ELISA. When indicated, anti-TSLP and anti-TNF Abs were added in culture. Data are means of duplicate determinations ± SD and represent one of five experiments (performed with four different CAFs and two different HDFs). Release of IL-13 significantly lower in the presence of an anti-TSLP Ab are indicated as: *, P < 0.05; **, 0.001 < P < 0.01 (determined by unpaired one-tailed Student’s t test).
Figure 4.
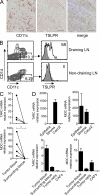
TSLP-conditioned DCs and Th2-attractant chemokines are present in vivo. (A and B) TSLP-conditioned DCs are present in the tumor and in draining but not in nondraining LNs. (A) Immunohistochemical analysis for CD11c+TSLPR+ cells in the tumor representative of tumor samples from 10 patients. Bars, 100 µm. (B) FACS analysis of CD11c+TSLPR+CD14− cells in draining (top) and corresponding nondraining (bottom) LNs representative of paired samples from four patients. Open histograms represent TSLPR staining; filled histograms represent the isotype control. Numbers indicate the percentage of gated (left) and positive (right) cells. (C and D) TARC/CCL17 and MDC/CCL22 mRNA expression in tumor and the corresponding surrounding tissue (C; each dot and corresponding triangle represent surgical samples from single patients), in tumor epithelial and stromal cells collected by LCM (D, top; representative of tumor samples from three patients), and in isolated and in vitro–cultured paired tumor cell lines and CAFs from the same patient (D, bottom; representative of tumor samples from three patients). Data in C and D are means of at least duplicate determinations ± SD. Responses significantly different are indicated as: *, P < 0.05 (determined by paired one-tailed Student’s t test).
Comment in
- Immunotherapy: TSLP fuels inflammation in breast and pancreatic tumors.
Schmitt CA. Schmitt CA. Nat Rev Clin Oncol. 2011 May;8(5):254. doi: 10.1038/nrclinonc.2011.40. Nat Rev Clin Oncol. 2011. PMID: 21451464 No abstract available. - Tumour immunology: TSLP drives human tumour progression.
Papatriantafyllou M. Papatriantafyllou M. Nat Rev Immunol. 2011 Apr;11(4):235. doi: 10.1038/nri2961. Nat Rev Immunol. 2011. PMID: 21553711 No abstract available. - TSLP1: a new piece in the puzzle of tumor-associated Th2-type inflammation.
Laderach DJ, Pesoa SA, Compagno D, Rabinovich GA. Laderach DJ, et al. Immunotherapy. 2011 Jun;3(6):713-7. doi: 10.2217/imt.11.67. Immunotherapy. 2011. PMID: 21668306 No abstract available.
Similar articles
- Thymic Stromal Lymphopoietin and Cancer: Th2-Dependent and -Independent Mechanisms.
Protti MP, De Monte L. Protti MP, et al. Front Immunol. 2020 Sep 16;11:2088. doi: 10.3389/fimmu.2020.02088. eCollection 2020. Front Immunol. 2020. PMID: 33042121 Free PMC article. Review. - The IL-1/IL-1 receptor axis and tumor cell released inflammasome adaptor ASC are key regulators of TSLP secretion by cancer associated fibroblasts in pancreatic cancer.
Brunetto E, De Monte L, Balzano G, Camisa B, Laino V, Riba M, Heltai S, Bianchi M, Bordignon C, Falconi M, Bondanza A, Doglioni C, Protti MP. Brunetto E, et al. J Immunother Cancer. 2019 Feb 13;7(1):45. doi: 10.1186/s40425-019-0521-4. J Immunother Cancer. 2019. PMID: 30760333 Free PMC article. - Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus.
Hanabuchi S, Ito T, Park WR, Watanabe N, Shaw JL, Roman E, Arima K, Wang YH, Voo KS, Cao W, Liu YJ. Hanabuchi S, et al. J Immunol. 2010 Mar 15;184(6):2999-3007. doi: 10.4049/jimmunol.0804106. Epub 2010 Feb 19. J Immunol. 2010. PMID: 20173030 Free PMC article. - Thymic stromal lymphopoietin receptor blockade reduces allergic inflammation in a cynomolgus monkey model of asthma.
Cheng DT, Ma C, Niewoehner J, Dahl M, Tsai A, Zhang J, Gonsiorek W, Apparsundaram S, Pashine A, Ravindran P, Jung J, Hang J, Allard J, Bitter H, Tribouley C, Narula S, Wilson S, Fuentes ME. Cheng DT, et al. J Allergy Clin Immunol. 2013 Aug;132(2):455-62. doi: 10.1016/j.jaci.2013.05.011. Epub 2013 Jun 26. J Allergy Clin Immunol. 2013. PMID: 23810153 - Expression and Regulation of Thymic Stromal Lymphopoietin and Thymic Stromal Lymphopoietin Receptor Heterocomplex in the Innate-Adaptive Immunity of Pediatric Asthma.
Lin SC, Cheng FY, Liu JJ, Ye YL. Lin SC, et al. Int J Mol Sci. 2018 Apr 18;19(4):1231. doi: 10.3390/ijms19041231. Int J Mol Sci. 2018. PMID: 29670037 Free PMC article. Review.
Cited by
- Thymic Stromal Lymphopoietin and Cancer: Th2-Dependent and -Independent Mechanisms.
Protti MP, De Monte L. Protti MP, et al. Front Immunol. 2020 Sep 16;11:2088. doi: 10.3389/fimmu.2020.02088. eCollection 2020. Front Immunol. 2020. PMID: 33042121 Free PMC article. Review. - Neoadjuvant therapy alters the immune microenvironment in pancreatic cancer.
Zhang H, Ye L, Yu X, Jin K, Wu W. Zhang H, et al. Front Immunol. 2022 Sep 26;13:956984. doi: 10.3389/fimmu.2022.956984. eCollection 2022. Front Immunol. 2022. PMID: 36225934 Free PMC article. Review. - Distinct T helper cell-mediated antitumor immunity: T helper 2 cells in focus.
Silva RCMC, Lopes MF, Travassos LH. Silva RCMC, et al. Cancer Pathog Ther. 2022 Nov 4;1(1):76-86. doi: 10.1016/j.cpt.2022.11.001. eCollection 2023 Jan. Cancer Pathog Ther. 2022. PMID: 38328613 Free PMC article. Review. - Immune correlates of therapy outcomes in women with cervical cancer treated with chemoradiotherapy: A systematic review.
Lakomy DS, Wu J, Lombe D, Papasavvas E, Msadabwe SC, Geng Y, Montaner LJ, Chiao E, Lin LL. Lakomy DS, et al. Cancer Med. 2021 Jul;10(13):4206-4220. doi: 10.1002/cam4.4017. Epub 2021 Jun 12. Cancer Med. 2021. PMID: 34117731 Free PMC article. - Construction of immunogenic cell death-related molecular subtypes and prognostic signature in colorectal cancer.
Yu C, Yang W, Tian L, Qin Y, Gong Y, Cheng W. Yu C, et al. Open Med (Wars). 2023 Nov 9;18(1):20230836. doi: 10.1515/med-2023-0836. eCollection 2023. Open Med (Wars). 2023. PMID: 38025525 Free PMC article.
References
- Arlt A., Vorndamm J., Müerköster S., Yu H., Schmidt W.E., Fölsch U.R., Schäfer H. 2002. Autocrine production of interleukin 1beta confers constitutive nuclear factor kappaB activity and chemoresistance in pancreatic carcinoma cell lines. Cancer Res. 62:910–916 - PubMed
- Atzeni F., Sarzi-Puttini P. 2009. Anti-cytokine antibodies for rheumatic diseases. Curr. Opin. Investig. Drugs. 10:1204–1211 - PubMed
- Bogiatzi S.I., Fernandez I., Bichet J.C., Marloie-Provost M.A., Volpe E., Sastre X., Soumelis V. 2007. Cutting edge: proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J. Immunol. 178:3373–3377 - PubMed
- Burris H.A., III, Moore M.J., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R., Cripps M.C., Portenoy R.K., Storniolo A.M., Tarassoff P., et al. 1997. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 15:2403–2413 - PubMed
- Curiel T.J., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P., Evdemon-Hogan M., Conejo-Garcia J.R., Zhang L., Burow M., et al. 2004. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10:942–949 10.1038/nm1093 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical