Crohn disease--associated adherent-invasive E. coli bacteria target mouse and human Peyer's patches via long polar fimbriae - PubMed (original) (raw)
. 2011 Mar;121(3):966-75.
doi: 10.1172/JCI44632. Epub 2011 Feb 21.
Affiliations
- PMID: 21339647
- PMCID: PMC3049390
- DOI: 10.1172/JCI44632
Crohn disease--associated adherent-invasive E. coli bacteria target mouse and human Peyer's patches via long polar fimbriae
Benoit Chassaing et al. J Clin Invest. 2011 Mar.
Abstract
Crohn disease (CD) is a multifactorial disease in which an abnormal immune response in the gastrointestinal (GI) tract leads to chronic inflammation. The small intestine, particularly the ileum, of patients with CD is colonized by adherent-invasive E. coli (AIEC)--a pathogenic group of E. coli able to adhere to and invade intestinal epithelial cells. As the earliest inflammatory lesions are microscopic erosions of the epithelium lining the Peyer's patches (PPs), we investigated the ability of AIEC bacteria to interact with PPs and the virulence factors involved. We found that AIEC bacteria could interact with mouse and human PPs via long polar fimbriae (LPF). An LPF-negative AIEC mutant was highly impaired in its ability to interact with mouse and human PPs and to translocate across monolayers of M cells, specialized epithelial cells at the surface of PPs. The prevalence of AIEC strains harboring the lpf operon was markedly higher in CD patients compared with controls. In addition, increased numbers of AIEC, but not LPF-deficient AIEC, bacteria were found interacting with PPs from Nod2(-/-) mice compared with WT mice. In conclusion, we have identified LPF as a key factor for AIEC to target PPs. This could be the missing link between AIEC colonization and the presence of early lesions in the PPs of CD patients.
Figures
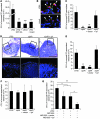
Figure 1. Interaction of AIEC bacteria with PPs.
(A) Interaction of bacteria with murine PPs placed in Ussing chambers after a 4-hour infection period, with or without anti-GP2 antibody (1 μg/ml) and with or without 0.5% methyl α-
d
-mannopyranoside (mann). When needed, PPs were preincubated with anti-GP2 antibody. (B) Confocal analysis of PP sections after labeling of AIEC LF82 with LPS O83 antibody (green), of M cells with anti-GP2 antibody (red), and DNA with Hoechst (blue). Scale bars: 20 μm. Arrowheads show clear colocalization of bacteria and M cells. (C) Interaction of wild-type LF82 and lpf mutant with murine PPs placed in Ussing chambers after a 4-hour infection period. (D) HES staining and confocal analysis of indicated areas after Cy3-EUB228 FISH staining to detect bacteria (red) and Hoechst to identify DNA (blue). Scale bars: 100 μm for HES staining and 20 μm for confocal analysis. Images in the bottom row correspond to the boxed regions in the top row. (E) In vivo interaction of wild-type LF82 and lpf mutant with murine PPs using ileal loop assay after a 4-hour infection period in the presence of 0.5% methyl α-
d
-mannopyranoside. (F) Interaction of wild-type LF82 and lpf mutant with murine small intestine mucosa without PPs after a 4-hour infection period in Ussing chambers. All results are expressed as numbers of mucosa-associated bacteria; each value is the mean ± SEM of at least 5 separate experiments. (G) Interaction of wild-type LF82 bacteria with murine PPs placed in Ussing chambers and coincubated with nonpathogenic MG1655 E. coli K-12 strain expressing or not expressing LPFLF82. *P < 0.05, **P < 0.01, ***P < 0.001.
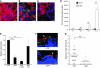
Figure 2. Involvement of LPF to promote interaction between AIEC LF82 bacteria and PPs.
(A) Quantification of intra-human PP-associated bacteria by confocal microscopy. Each value is the mean ± SEM of 2–4 separate experiments, with 3–5 sections studied for each experiment. **P < 0.01. (B) Representative confocal photomicrographs of uptake of bacteria across human FAE. Scale bars: 100 μm for HES staining and 50 μm for confocal analysis. Images in the bottom row correspond to the boxed regions in the top row. See the Figure 1D legend for staining.
Figure 3. LPF are involved in the ability of AIEC strain LF82 to target M cells.
Interaction of AIEC bacteria LF82 (A and B) and LF82-ΔlpfA isogenic mutant (C) with Caco-2-cl1 (A) or M-like cell (B and C) monolayers in the presence of 0.5% methyl α-
d
-mannopyranoside. Phalloidin-TRITC labeling of F-actin (red), anti-O83 antibody labeling of LF82 bacteria (green), and Hoechst labeling of DNA (blue) were used. Confocal photomicrographs of interaction of bacteria with in vitro M cells are representative of 3 separate experiments. Scale bars: 25 μm. (D) Translocation across Caco-2-cl1 or M-like cell monolayers. Results are expressed as CFU of translocated bacteria. Each value is the mean ± SEM of at least 5 separate experiments. (E) Translocation of LF82 bacteria and LF82-ΔlpfA isogenic mutant across M-like cell monolayers after 4 hours infection. Results are expressed as translocated bacteria relative to those obtained for strain LF82, taken as 1. (F) Confocal analysis of murine PP sections after labeling of AIEC LF82 with LPS O83 antibody (green), of M cell with UEA-1 TRITC (red), and DNA with Hoechst (blue). Scale bars: 20 μm. Arrowheads indicate UEA-1–positive cells. (G) Quantification of murine M cell–associated bacteria by confocal microscopy analysis. Bars represent the mean. **P < 0.01, ***P < 0.001.
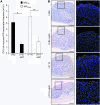
Figure 4. Comparison of ability of LPF_Shigella_ and LPFLF82 bacteria to interact with murine PPs.
(A) Quantification of murine PP-associated bacteria for AIEC strains LF82 and LF110 and corresponding Δ_lpfA_ mutants. See the legend to Figure 1C. Each value is the mean ± SEM of at least 5 separate experiments. (B) Visualization of bacteria interacting with murine isolated PPs. Scale bars: 100 μm for HES staining and 50 μm for confocal analysis. Images in the right column correspond to the boxed regions in the left row. See the legend to Figure 1D. *P < 0.05, **P < 0.01.
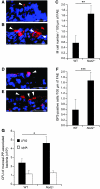
Figure 5. LPF-dependent increased interaction of AIEC LF82 bacteria with Nod2–/– PPs.
Visualization (A, B, D, and E) and quantification (C and F) of UEA-1 TRITC–stained M cells (red) (A–C) and GP2-positive cells (red) (D–F) present in FAE of wild-type (A and D) and Nod2–/– (B and E) mice after cryostat section. Scale bars: 20 μm. Arrowheads indicate UEA-1– or GP2-positive cells. In C and F, results are expressed as number of M cells present in 100 μm of FAE. Each value is the mean ± SEM of 6 separate experiments, with 5–6 sections studied for each experiment. (G) Ability of wild-type LF82 bacteria and LPF-negative mutant to interact with murine PPs from wild-type and Nod2–/– mice. See the legend to Figure 1C. Each value is the mean ± SEM of at least 5 separate experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Comment in
- Adherent-invasive E. coli in Crohn disease: bacterial "agent provocateur".
Strober W. Strober W. J Clin Invest. 2011 Mar;121(3):841-4. doi: 10.1172/JCI46333. Epub 2011 Feb 21. J Clin Invest. 2011. PMID: 21339637 Free PMC article. - Crohn's disease: Adherent-invasive Escherichia coli target Peyer's patches.
Greenhill C. Greenhill C. Nat Rev Gastroenterol Hepatol. 2011 May;8(5):246. doi: 10.1038/nrgastro.2011.43. Epub 2011 Mar 29. Nat Rev Gastroenterol Hepatol. 2011. PMID: 21451486 No abstract available.
Similar articles
- GipA Factor Supports Colonization of Peyer's Patches by Crohn's Disease-associated Escherichia Coli.
Vazeille E, Chassaing B, Buisson A, Dubois A, de Vallée A, Billard E, Neut C, Bommelaer G, Colombel JF, Barnich N, Darfeuille-Michaud A, Bringer MA. Vazeille E, et al. Inflamm Bowel Dis. 2016 Jan;22(1):68-81. doi: 10.1097/MIB.0000000000000609. Inflamm Bowel Dis. 2016. PMID: 26512715 - Bile salts induce long polar fimbriae expression favouring Crohn's disease-associated adherent-invasive Escherichia coli interaction with Peyer's patches.
Chassaing B, Etienne-Mesmin L, Bonnet R, Darfeuille-Michaud A. Chassaing B, et al. Environ Microbiol. 2013 Feb;15(2):355-71. doi: 10.1111/j.1462-2920.2012.02824.x. Epub 2012 Jul 13. Environ Microbiol. 2013. PMID: 22789019 - Adherent-invasive E. coli in Crohn disease: bacterial "agent provocateur".
Strober W. Strober W. J Clin Invest. 2011 Mar;121(3):841-4. doi: 10.1172/JCI46333. Epub 2011 Feb 21. J Clin Invest. 2011. PMID: 21339637 Free PMC article. - Pathogenesis of adherent-invasive Escherichia coli.
Smith EJ, Thompson AP, O'Driscoll A, Clarke DJ. Smith EJ, et al. Future Microbiol. 2013 Oct;8(10):1289-300. doi: 10.2217/fmb.13.94. Future Microbiol. 2013. PMID: 24059919 Review. - Adherent-invasive Escherichia coli in inflammatory bowel disease.
Rolhion N, Darfeuille-Michaud A. Rolhion N, et al. Inflamm Bowel Dis. 2007 Oct;13(10):1277-83. doi: 10.1002/ibd.20176. Inflamm Bowel Dis. 2007. PMID: 17476674 Review.
Cited by
- Analysis of the σE regulon in Crohn's disease-associated Escherichia coli revealed involvement of the waaWVL operon in biofilm formation.
Chassaing B, Garénaux E, Carriere J, Rolhion N, Guérardel Y, Barnich N, Bonnet R, Darfeuille-Michaud A. Chassaing B, et al. J Bacteriol. 2015 Apr;197(8):1451-65. doi: 10.1128/JB.02499-14. Epub 2015 Feb 9. J Bacteriol. 2015. PMID: 25666140 Free PMC article. - Colonization of larval zebrafish (Danio rerio) with adherent-invasive Escherichia coli prevents recovery of the intestinal mucosa from drug-induced enterocolitis.
Flores E, Dutta S, Bosserman R, van Hoof A, Krachler A-M. Flores E, et al. mSphere. 2023 Dec 20;8(6):e0051223. doi: 10.1128/msphere.00512-23. Epub 2023 Nov 16. mSphere. 2023. PMID: 37971273 Free PMC article. - Microbiota-gut-brain axis and its affect inflammatory bowel disease: Pathophysiological concepts and insights for clinicians.
Sinagra E, Utzeri E, Morreale GC, Fabbri C, Pace F, Anderloni A. Sinagra E, et al. World J Clin Cases. 2020 Mar 26;8(6):1013-1025. doi: 10.12998/wjcc.v8.i6.1013. World J Clin Cases. 2020. PMID: 32258072 Free PMC article. Review. - Diversity and Adaptations of Escherichia coli Strains: Exploring the Intestinal Community in Crohn's Disease Patients and Healthy Individuals.
Siniagina MN, Markelova MI, Boulygina EA, Laikov AV, Khusnutdinova DR, Abdulkhakov SR, Danilova NA, Odintsova AH, Abdulkhakov RA, Grigoryeva TV. Siniagina MN, et al. Microorganisms. 2021 Jun 15;9(6):1299. doi: 10.3390/microorganisms9061299. Microorganisms. 2021. PMID: 34203637 Free PMC article. - Effect of α-Hemolysin Producing E. coli in Two Different Mouse Strains in a DSS Model of Inflammatory Bowel Disease.
Mirsepasi-Lauridsen HC, Struve C, Petersen AM, Krogfelt KA. Mirsepasi-Lauridsen HC, et al. Microorganisms. 2020 Dec 11;8(12):1971. doi: 10.3390/microorganisms8121971. Microorganisms. 2020. PMID: 33322398 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials