Lymphatic endothelial heparan sulfate deficiency results in altered growth responses to vascular endothelial growth factor-C (VEGF-C) - PubMed (original) (raw)
Lymphatic endothelial heparan sulfate deficiency results in altered growth responses to vascular endothelial growth factor-C (VEGF-C)
Xin Yin et al. J Biol Chem. 2011.
Abstract
Growth and remodeling of lymphatic vasculature occur during development and during various pathologic states. A major stimulus for this process is the unique lymphatic vascular endothelial growth factor-C (VEGF-C). Other endothelial growth factors, such as fibroblast growth factor-2 (FGF-2) or VEGF-A, may also contribute. Heparan sulfate is a linear sulfated polysaccharide that facilitates binding and action of some vascular growth factors such as FGF-2 and VEGF-A. However, a direct role for heparan sulfate in lymphatic endothelial growth and sprouting responses, including those mediated by VEGF-C, remains to be examined. We demonstrate that VEGF-C binds to heparan sulfate purified from primary lymphatic endothelia, and activation of lymphatic endothelial Erk1/2 in response to VEGF-C is reduced by interference with heparin or pretreatment of cells with heparinase, which destroys heparan sulfate. Such treatment also inhibited phosphorylation of the major VEGF-C receptor VEGFR-3 upon VEGF-C stimulation. Silencing lymphatic heparan sulfate chain biosynthesis inhibited VEGF-C-mediated Erk1/2 activation and abrogated VEGFR-3 receptor-dependent binding of VEGF-C to the lymphatic endothelial surface. These findings prompted targeting of lymphatic N-deacetylase/N-sulfotransferase-1 (Ndst1), a major sulfate-modifying heparan sulfate biosynthetic enzyme. VEGF-C-mediated Erk1/2 phosphorylation was inhibited in Ndst1-silenced lymphatic endothelia, and scratch-assay responses to VEGF-C and FGF-2 were reduced in Ndst1-deficient cells. In addition, lymphatic Ndst1 deficiency abrogated cell-based growth and proliferation responses to VEGF-C. In other studies, lymphatic endothelia cultured ex vivo from Ndst1 gene-targeted mice demonstrated reduced VEGF-C- and FGF-2-mediated sprouting in collagen matrix. Lymphatic heparan sulfate may represent a novel molecular target for therapeutic intervention.
Figures
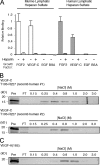
FIGURE 1.
VEGF-C binds to lymphatic endothelial heparan sulfate. A, oil granuloma/lymphangioma lesions were induced in wild type mice, and cultured lymphatic endothelial cells from lesions were radiolabeled with 35SO4. [35S]Heparan sulfate from the cells was purified and incubated with recombinant mouse VEGF-C (mature, fully processed 125-amino acid sequence, described under “Experimental Procedures”), followed by passage over nitrocellulose membrane filters. Membrane-bound complexes of [35S]heparan sulfate and protein collected on the filters were quantified and plotted relative to that of FGF-2 (open bars, left). Binding was also tested in the presence of heparin as a competitor. Controls included binding to EGF and BSA. The same experiment was carried out using recombinant human VEGF-C (mature, fully processed 125-amino acid sequence, described under “Experimental Procedures”) and [35S]heparan sulfate from primary human lung lymphatic endothelial cells (filled bars, right; p = 0.01 for FGF-2 versus VEGF-C; average of four experiments). B, to assess binding of the same recombinant human VEGF-C protein (designated recomb human #1) to commercial heparin, affinity chromatography using heparin-Sepharose columns was carried out. Fractions collected over the indicated NaCl step concentration range were run on SDS-PAGE with silver staining to reveal the protein elution profile. The level to which native protein migrates on the gel is shown at left (Pre column sample in 1st lane with kDa range as indicated); column flow-through (FT) is shown in 2nd lane; and elution profile (subsequent lanes) is shown to the right. For comparison, heparin affinity chromatography was carried out for another commercial recombinant human VEGF-C preparation (mature, fully processed 125-amino acid sequence, recomb human #2, shown below). C, binding of recombinant human VEGF-A (VEGF165) was also examined.
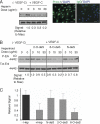
FIGURE 2.
VEGF-C-mediated Erk1/2 activation in lymphatic endothelium is inhibited by the presence of heparin, with unique responses in the presence of sulfate-modified heparinoids. A, phosphorylation of Erk1/2 (p44/42) in serum-starved human primary lung lymphatic endothelial cells in response to treatment with recombinant human VEGF-C was measured in the absence/presence of increasing commercial heparin concentration. Lymphatic endothelial cell purity was confirmed by immunostaining to detect nuclear expression of Prox-1 (right photomicrographs; with IgG isotype-matched control antibody stain to right; bar, 50 μm). Maximum phosphorylation was noted in the absence of heparin (+VEGF-C, heparin = 0). Phospho/total signal intensities were quantified by densitometry and normalized relative to that of maximum stimulation (Signal Relative to Max, below panel). Preliminary testing showed that marked inhibition was achieved at heparin doses >1 μg/ml. B, effects of _N_-desulfated (N-deS), 2-_O_-desulfated (2-O-deS), or 6-_O_-desulfated (6-O-deS) heparin species were examined at the same doses as that used for unmodified commercial heparin in A. C, quantitative assessment for responses in the presence of heparin versus sulfate-modified heparinoids dosed at 10 μg/ml is shown (graph). Values represent the densitometry ratio of phospho/total Erk for VEGF-C-mediated stimulation in the presence of either heparin (+ Hep) or each of the respective heparinoids normalized to the signal for stimulation in the absence of heparin (− Hep). Graph shows mean of four experiments. *, p < 0.001 for + heparin versus − heparin; **, p < 0.001 for 6-_O_-deS versus − heparin; differences in means for _N_-deS versus − heparin or 2-_O_-deS versus − heparin were not significant).
FIGURE 3.
Heparinase-mediated ablation of lymphatic endothelial cell-surface heparan sulfate inhibits growth signaling and receptor activation in response to VEGF-C. A, phosphorylation of Erk1/2 (p44/42) in serum-starved primary human lung lymphatic endothelial cells was measured before/after growth factor stimulation. Responses to stimulation with recombinant human VEGF-C were measured for cells pretreated with or without heparinase (used to destroy cell-surface heparan sulfate). Total Erk for each panel is shown below respective phospho-Erk panels. Ratios of phospho/total Erk band intensities for each condition were quantified by densitometry and normalized to the respective base-line phospho/total Erk ratio (shown in parentheses below each panel). B, responses to stimulation by FGF-2 (left panels) as well as the non-heparin-binding factor EGF (right panels) pretreated with or without heparinase were also examined. C, VEGFR-3 receptor phosphorylation in response to VEGF-C was examined in the absence/presence of heparan sulfate elimination by heparinase. Receptor was immunoprecipitated from lysates of either serum-starved LEC (baseline) or the same cells preincubated in the absence/presence of heparinase and stimulated with VEGF-C. Immunoblotting with anti-phosphotyrosine was carried out and revealed a phosphorylated product in response to stimulation with VEGF-C (WB: P-Tyr; middle lane), consistent with phosphorylated VEGFR-3. The response of heparinase-treated cells is shown in the right lane. Total receptor in the original lysates (TL) is shown below (WB: VEGFR-3). Graph (right) shows mean signal intensities normalized to base-line signal for n = 3 experiments (±S.E.). *, p = 0.001 for difference relative to normalized base-line signal; **, p = 0.009 for difference between mean heparanase and mean VEGF-C values.
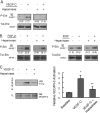
FIGURE 4.
Silencing of lymphatic endothelial heparan sulfate chain biosynthesis inhibits VEGF-C-dependent Erk1/2 activation and reduces receptor-dependent binding of VEGF-C. A, cultured primary human lymphatic endothelia were transfected with siRNA targeting XylT2 (siXylT2) or with scrambled duplex RNA (siDS) as a control. Under each condition, phosphorylation of Erk1/2 in serum-starved cells before/after recombinant human VEGF-C exposure was examined by Western blotting. Total Erk for each condition is shown below respective phospho-Erk lanes. Ratios of phospho/total Erk band intensities for each condition were quantified and normalized to the respective base-line ratio (values in parentheses). Graph (below) shows mean signal intensities for n = 4 experiments (±S.E.). B, in separate experiments, exposure of mock-transfected (siDS) control cells to exogenous recombinant human VEGF-C followed by washing resulted in the engagement of numerous cell-surface VEGF-C-VEGFR-3 complexes (red dots, top right photomicrograph; siDS +VEGF-C), as measured through PLA. There was minimal endogenous signal at base line, prior to exogenous VEGF-C application (top left; siDS). Exposure of siXylT2-targeted cells to exogenous VEGF-C resulted in minimal complex formation over base line (bottom photomicrographs; bar, 50 μm). C, quantified results, showing the fold increase in fluorescent signal for each condition indexed to base-line signal for siDS control. Also shown on the graph (right) is the mean signal achieved by cells treated with siRNA targeting VEGFR-3 and exposed to exogenous VEGF-C (labeled siVR3+VEGF-C). *, p values for difference between siDS + VEGF-C and the following: siDS base line (p < 0.001); siXylT2 + VEGF-C (p = 0.001); siVR3 + VEGF-C (p = 0.004). **, p = 0.04 for difference in base-line signals (siDS versus siXylT2).
FIGURE 5.
Targeting lymphatic endothelial heparan sulfate chain biosynthesis abrogates VEGF-C-dependent gap filling and growth responses in cultured cell systems. Monolayers of serum-starved human lymphatic endothelia transfected with siRNA targeting XylT2 (siXylT2) or with scrambled duplex RNA (siDS; control) were scratched to create a gap in the center at time 0. The ability of cells to fill the gap in response to VEGF-C was examined. A, graphs show quantitative data for percentage of gap filling over time in the presence/absence of VEGF-C by control cells (left panel) and by siXylT2-targeted cells (right panel). Photomicrographs below show gap (with dotted gap boundaries) within confluent cell layers at time 0, and degree of gap filling at 48 h in the presence of VEGF-C (bar, 0.5 mm). *, p = 0.009 and **, p = 0.003 for difference in mean responses by VEGF-C-stimulated versus un-stimulated control (siDS) cells at 24 h and 48 h, respectively. (Graph, mean of n = 3 trials.). B, cell viability assays demonstrate growth responses of control (siDS), siXylT2-, and siNdst1-targeted lymphatic endothelia in response to 24 h of exposure to VEGF-C. *, p < 0.001 for the difference in mean growth responses by control (siDS) cells stimulated ± VEGF-C for 24 h. Other differences were not significant.
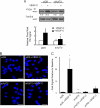
FIGURE 6.
Targeting lymphatic endothelial Ndst1 results in reduced growth and sprouting responses to VEGF-C and FGF-2. A, serum-starved human lymphatic endothelia were transfected with siRNA targeting Ndst1 (siNdst1) or with scrambled duplex RNA (siDS; control). Phosphorylation of Erk1/2 before/after VEGF-C exposure was examined by Western blotting. Total Erk for each condition is shown. Ratios of phospho/total Erk band intensities for each condition were quantified and normalized to the respective base-line ratio (values in parentheses). Graph (right) shows mean signal intensities for n = 4 experiments (±S.E.). B, ability of the siRNA-targeted lymphatic endothelia to fill a gap made between cells in a scratch assay was examined over 48 h in the presence/absence of growth factor. Photomicrographs show gap (with dotted gap boundaries) within confluent cell layers at time 0, and at 48 h in the presence of VEGF-C (bar, 0.5 mm). Control siDS cells partially filled the gap over 48 h (upper photomicrographs), whereas response by siNdst1-targeted cells is shown below. Mean values for responses in the presence/absence of VEGF-C were graphed (top). *, p = 0.01 for VEGF-C versus un-stimulated cells at 48 h. Graphs for assays carried out in the presence of the cell proliferation inhibitor mitomycin-C are also shown (labeled +Mito-C). The scratch assay was also carried out for cell stimulation in the presence/absence of FGF-2 (graphs shown below photomicrographs). *, p = 0.008 for FGF-2 versus unstimulated cells at 24 h; **, p < 0.001 for FGF-2 versus unstimulated cells at 48 h. (Graphs, mean of n = 3 trials.) C, lymphatic endothelia were isolated from oil granuloma/lymphangioma lesions generated in Ndst1f/fTekCre(+) mutant mice and Cre(−) littermates, with podoplanin and LYVE-1 expression shown by FACS. D, sprouting responses in collagen upon stimulation with recombinant VEGF-C or FGF-2 were examined. Significant responses to growth factor were noted for Cre(−) wild type cells (*, p = 0.003, and **, p = 0.008 for respective VEGF-C and FGF-2 responses versus base-line responses in the absence of growth factor, None). Mutant responses (right) were not significantly different from base line. Sulfation of heparan sulfate disaccharides purified from mutant versus wild type cells was determined by liquid chromatography/mass spectrometry and quantified as a percentage of total disaccharides (bottom graph). The detected disaccharide species are listed (x axis) according to published nomenclature (21), with D0A0 (left-most bars) indicating the percentages of unsulfated disaccharides.
Similar articles
- Functional Importance of a Proteoglycan Coreceptor in Pathologic Lymphangiogenesis.
Johns SC, Yin X, Jeltsch M, Bishop JR, Schuksz M, El Ghazal R, Wilcox-Adelman SA, Alitalo K, Fuster MM. Johns SC, et al. Circ Res. 2016 Jul 8;119(2):210-21. doi: 10.1161/CIRCRESAHA.116.308504. Epub 2016 May 25. Circ Res. 2016. PMID: 27225479 Free PMC article. - A critical role for lymphatic endothelial heparan sulfate in lymph node metastasis.
Yin X, Truty J, Lawrence R, Johns SC, Srinivasan RS, Handel TM, Fuster MM. Yin X, et al. Mol Cancer. 2010 Dec 20;9:316. doi: 10.1186/1476-4598-9-316. Mol Cancer. 2010. PMID: 21172016 Free PMC article. - Molecular mechanisms of lymphangiogenesis.
Takahashi M, Yoshimoto T, Kubo H. Takahashi M, et al. Int J Hematol. 2004 Jul;80(1):29-34. doi: 10.1532/ijh97.04042. Int J Hematol. 2004. PMID: 15293565 Review. - Lymphangiogenic growth factors, receptors and therapies.
Lohela M, Saaristo A, Veikkola T, Alitalo K. Lohela M, et al. Thromb Haemost. 2003 Aug;90(2):167-84. doi: 10.1160/TH03-04-0200. Thromb Haemost. 2003. PMID: 12888864 Review.
Cited by
- Langerhans cells regulate immunity in adulthood by regulating postnatal dermal lymphatic development.
Sim JH, Bell R, Feng Z, Chyou S, Shipman WD, Kataru RP, Ivashkiv L, Mehrara B, Lu TT. Sim JH, et al. bioRxiv [Preprint]. 2024 Jul 16:2024.07.12.603312. doi: 10.1101/2024.07.12.603312. bioRxiv. 2024. PMID: 39071369 Free PMC article. Preprint. - Dysregulation of Lymphatic Endothelial VEGFR3 Signaling in Disease.
Kuonqui K, Campbell AC, Sarker A, Roberts A, Pollack BL, Park HJ, Shin J, Brown S, Mehrara BJ, Kataru RP. Kuonqui K, et al. Cells. 2023 Dec 28;13(1):68. doi: 10.3390/cells13010068. Cells. 2023. PMID: 38201272 Free PMC article. Review. - Heparanase and the hallmarks of cancer.
Jayatilleke KM, Hulett MD. Jayatilleke KM, et al. J Transl Med. 2020 Nov 30;18(1):453. doi: 10.1186/s12967-020-02624-1. J Transl Med. 2020. PMID: 33256730 Free PMC article. Review. - Functional Cellular Anti-Tumor Mechanisms are Augmented by Genetic Proteoglycan Targeting.
Gupta P, Johns SC, Kim SY, El Ghazal R, Zuniga EI, Fuster MM. Gupta P, et al. Neoplasia. 2020 Feb;22(2):86-97. doi: 10.1016/j.neo.2019.11.003. Epub 2019 Dec 30. Neoplasia. 2020. PMID: 31896526 Free PMC article. - Leucocyte Trafficking via the Lymphatic Vasculature- Mechanisms and Consequences.
Jackson DG. Jackson DG. Front Immunol. 2019 Mar 14;10:471. doi: 10.3389/fimmu.2019.00471. eCollection 2019. Front Immunol. 2019. PMID: 30923528 Free PMC article. Review.
References
- Alitalo K., Tammela T., Petrova T. V. (2005) Nature 438, 946–953 - PubMed
- Liersch R., Detmar M. (2007) Thromb. Haemost. 98, 304–310 - PubMed
- Wissmann C., Detmar M. (2006) Clin. Cancer Res. 12, 6865–6868 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous