MicroRNAs: a potential interface between the circadian clock and human health - PubMed (original) (raw)
MicroRNAs: a potential interface between the circadian clock and human health
Katelin F Hansen et al. Genome Med. 2011.
Abstract
The biochemical activity of a stunning diversity of cell types and organ systems is shaped by a 24-hour (circadian) clock. This rhythmic drive to a good deal of the transcriptome (up to 15% of all coding genes) imparts circadian modulation over a wide range of physiological and behavioral processes (from cell division to cognition). Further, dysregulation of the clock has been implicated in the pathogenesis of a large and diverse array of disorders, such as hypertension, cancer and depression. Indeed, the possibility of utilizing therapeutic approaches that target clock physiology (that is, chronotherapy) has gained broad interest. However, a deeper understanding of the underlying molecular mechanisms that modulate the clock, and give rise to organ-specific clock transcriptomes, will be required to fully realize the power of chronotherapies. Recently, microRNAs have emerged as significant players in circadian clock timing, thus raising the possibility that clock-controlled microRNAs could contribute to disorders of the human circadian timing system. Here, we highlight recent work revealing a key role for microRNAs in clock physiology, and discuss potential approaches to unlocking their utility as effectors of circadian physiology and pathophysiology.
Figures
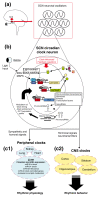
Figure 1
Schematic overview of molecular circadian clock timing. (a) Suprachiasmatic nucleus (SCN; red circles) located within the ventral hypothalamus. The two nuclei serve as the master circadian clocks, orchestrating rhythmicity and phasing of ancillary clocks found in peripheral organ systems (c1) and throughout the brain (c2). Entrainment of the SCN clock to light is mediated by a direct projection from the retina. (b) The molecular clock feedback loop is centered on the rhythmic expression of the period (per1/2) and cryptochrome (cry1/2) gene families. PERIOD and CRY dimers translocate to the nucleus and negatively regulate their own production via suppression of CLOCK (CLK)- and BMAL1-mediated transcription. Casein kinase 1 (CK1)-mediated phosphorylation triggers degradation of PER proteins, thus relieving transcriptional repression and, in turn, allowing for robust 24-hour clock gene cycling. In addition to the core clock genes, a large fraction of the transcriptome is under the direct or indirect influence of the circadian clock. The clock-controlled transcriptome (CCT) within the SCN includes hormones, kinases, transcription factors and microRNAs. Many of these gene products serve as phasing cues to ancillary oscillators, or function as feedback regulators of the core molecular clock. (c1) Clock-regulated microRNA expression in the liver: denotation of several rhythmically expressed microRNAs that are either predicted (that is, miR181 d and miR191 [69]) or have been shown (that is, miR192/194 [71]) to target components of the core molecular clock. MiR122 has been shown to modulate clock-controlled gene expression involved in hepatic cholesterol and lipid metabolism [72,74]. In total, these data strongly indicate that microRNAs play a central role in sculpting the circadian gene expression profile and, in turn, regulate associated physiological and behavioral processes.
Similar articles
- Chronopharmacological strategies focused on chrono-drug discovery.
Ohdo S, Koyanagi S, Matsunaga N. Ohdo S, et al. Pharmacol Ther. 2019 Oct;202:72-90. doi: 10.1016/j.pharmthera.2019.05.018. Epub 2019 Jun 5. Pharmacol Ther. 2019. PMID: 31173839 Review. - Circadian system microRNAs - Role in the development of cardiovascular diseases.
Škrlec I. Škrlec I. Adv Protein Chem Struct Biol. 2023;137:225-267. doi: 10.1016/bs.apcsb.2023.02.004. Epub 2023 Feb 25. Adv Protein Chem Struct Biol. 2023. PMID: 37709378 Review. - A rhythmic placenta? Circadian variation, clock genes and placental function.
Waddell BJ, Wharfe MD, Crew RC, Mark PJ. Waddell BJ, et al. Placenta. 2012 Jul;33(7):533-9. doi: 10.1016/j.placenta.2012.03.008. Epub 2012 Apr 22. Placenta. 2012. PMID: 22525887 Review. - mTOR Signaling and Entrainment of the Mammalian Circadian Clock.
Cao R, Obrietan K. Cao R, et al. Mol Cell Pharmacol. 2010;2(4):125-130. doi: 10.4255/mcpharmacol.10.17. Mol Cell Pharmacol. 2010. PMID: 21274417 Free PMC article. - MicroRNAs modulate core-clock gene expression in pancreatic islets during early postnatal life in rats.
Jacovetti C, Rodriguez-Trejo A, Guay C, Sobel J, Gattesco S, Petrenko V, Saini C, Dibner C, Regazzi R. Jacovetti C, et al. Diabetologia. 2017 Oct;60(10):2011-2020. doi: 10.1007/s00125-017-4348-6. Epub 2017 Jul 4. Diabetologia. 2017. PMID: 28674733
Cited by
- Dicer expression exhibits a tissue-specific diurnal pattern that is lost during aging and in diabetes.
Yan Y, Salazar TE, Dominguez JM 2nd, Nguyen DV, Li Calzi S, Bhatwadekar AD, Qi X, Busik JV, Boulton ME, Grant MB. Yan Y, et al. PLoS One. 2013 Nov 7;8(11):e80029. doi: 10.1371/journal.pone.0080029. eCollection 2013. PLoS One. 2013. PMID: 24244599 Free PMC article. - Abnormal Expression of MicroRNAs Induced by Chronic Unpredictable Mild Stress in Rat Hippocampal Tissues.
Zhou M, Wang M, Wang X, Liu K, Wan Y, Li M, Liu L, Zhang C. Zhou M, et al. Mol Neurobiol. 2018 Feb;55(2):917-935. doi: 10.1007/s12035-016-0365-6. Epub 2017 Jan 12. Mol Neurobiol. 2018. PMID: 28083819 - MicroRNAs shape the neuronal landscape.
McNeill E, Van Vactor D. McNeill E, et al. Neuron. 2012 Aug 9;75(3):363-79. doi: 10.1016/j.neuron.2012.07.005. Neuron. 2012. PMID: 22884321 Free PMC article. Review. - Diurnal oscillations in human salivary microRNA and microbial transcription: Implications for human health and disease.
Hicks SD, Khurana N, Williams J, Dowd Greene C, Uhlig R, Middleton FA. Hicks SD, et al. PLoS One. 2018 Jul 18;13(7):e0198288. doi: 10.1371/journal.pone.0198288. eCollection 2018. PLoS One. 2018. PMID: 30020932 Free PMC article. - Adaptation of molecular circadian clockwork to environmental changes: a role for alternative splicing and miRNAs.
Bartok O, Kyriacou CP, Levine J, Sehgal A, Kadener S. Bartok O, et al. Proc Biol Sci. 2013 Jul 3;280(1765):20130011. doi: 10.1098/rspb.2013.0011. Print 2013 Aug 22. Proc Biol Sci. 2013. PMID: 23825200 Free PMC article. Review.
References
- Schwartz WJ. A clinician's primer on the circadian clock: its localization, function, and resetting. Adv Intern Med. 1993;38:81–106. - PubMed
LinkOut - more resources
Full Text Sources