Role of endosomes in simian virus 40 entry and infection - PubMed (original) (raw)
Role of endosomes in simian virus 40 entry and infection
Sabrina Engel et al. J Virol. 2011 May.
Erratum in
- Correction for Engel et al., "Role of Endosomes in Simian Virus 40 Entry and Infection".
Engel S, Heger T, Mancini R, Herzog F, Kartenbeck J, Hayer A, Helenius A. Engel S, et al. J Virol. 2017 Sep 27;91(20):e01059-17. doi: 10.1128/JVI.01059-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28956779 Free PMC article. No abstract available.
Abstract
After binding to its cell surface receptor ganglioside GM1, simian virus 40 (SV40) is endocytosed by lipid raft-mediated endocytosis and slowly transported to the endoplasmic reticulum, where partial uncoating occurs. We analyzed the intracellular pathway taken by the virus in HeLa and CV-1 cells by using a targeted small interfering RNA (siRNA) silencing screen, electron microscopy, and live-cell imaging as well as by testing a variety of cellular inhibitors and other perturbants. We found that the virus entered early endosomes, late endosomes, and probably endolysosomes before reaching the endoplasmic reticulum and that this pathway was part of the infectious route. The virus was especially sensitive to a variety of perturbations that inhibited endosome acidification and maturation. Contrary to our previous models, which postulated the passage of the virus through caveolin-rich organelles that we called caveosomes, we conclude that SV40 depends on the classical endocytic pathway for infectious entry.
Figures
Fig. 1.
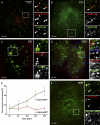
Internalized SV40 colocalizes with endosomal markers (see also Movies S1 to S3 in the supplemental material). (A) CV-1 cells were incubated with SV40-AF488 for 120 min at 37°C. Cells were mixed and immunostained with antibodies against the EE marker EEA1. A confocal section was imaged using the Zeiss LSM 510 confocal microscope system. (B to D) Fluorescently labeled SV40 was added to CV-1 cells transfected with different XFP-tagged endosomal markers and imaged live with a spinning-disc confocal microscope (except C, which was imaged with the Zeiss LSM 510 system) at the indicated time points. (E) Percentage of viruses colocalizing with the markers Rab7-EGFP and Rab5-mRFP at different times postwarming. It was calculated from images such as those shown in D. Error bars are standard errors of the means (SEM) of data from each time point for 5 to 15 cells from three different experiments, with an average of 50 to 100 particles per cell. (F) Same as A, but cells were incubated with SV40-AF594 and stained against the LE marker LAMP1.
Fig. 2.
Colocalization of internalized SV40 with EE and LE markers (see also Movie S4 in the supplemental material). (A and B) SV40-AF647 was added to CV-1 cells transfected with Rab7-mRFP and LAMP1-EGFP or Rab9-EYFP and imaged with a spinning-disc confocal microscope at 153 min postwarming. (C) Fluorescently labeled SV40 internalized into CV-1 cells transfected with the constitutively active mutant Rab5Q79L-EGFP and imaged live with a confocal microscope at 160 min postwarming. (D) Fluorescently labeled dextran was added to CV-1 cells for 2 h, incubated overnight, and subjected to SV40-AF647 for binding on ice for 2 h. At 240 min after warming to 37°C, cells were imaged with a confocal microscope.
Fig. 3.
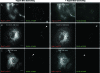
SV40 enters endosomal structures and the ER. (A) Purified SV40 visualized by negative staining. (B to G) Purified viruses were incubated with CV-1 cells at different times postwarming, fixed, embedded in plastic, sectioned, and imaged. Arrows point toward single virus particles. Viruses are seen as single particles (A) and in invaginations of the plasma membrane (B), endosomal structures (C to F), and smooth regions of the ER (G). The letter E in E stands for “endosome.” Scale bars indicate 200 nm.
Fig. 4.
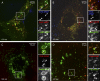
Acid dependence of internalization and infection. (A) CV-1 cells were pretreated for 1 h with 100 nM bafilomycin A1 (Baf), 20 mM NH4Cl, or 10 μM monensin (Mon) and were infected with SV40 for 24 h (MOI of 1) in the continued presence of the drugs. The infection level was determined by the immunostaining of the SV40 T antigen and flow cytometry and was normalized to drug-free controls. (B) Cells were infected with SV40 (MOI of 1), and Baf was added to the cells at different times. Infection was determined as described above for A. (C) Same as A but with mouse fibroblast caveolin-1 knockout cells and Baf. Data shown represent means ± SEM of data from three independent experiments, each with triplicate samples (A to C). (D) Percentages of SV40 particles internalized in the presence of NH4Cl, Baf, and Mon. Error bars indicate SEM of each time point for 5 to 15 cells from three different experiments, with an average of 50 to 100 particles per cell. (E to G) EM images of CV-1 cells incubated with SV40 particles in the presence of Mon (E and F) and Baf (G and H) at different times postwarming. Arrows point toward virus particles. Scale bars indicate 200 nm.
Fig. 5.
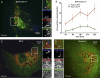
Internalization assay. (A) CV-1 cells were transfected with Rab7-mRFP 20 h before the addition of fluorescently labeled SV40. Cells were incubated in the cold for 1 h, unbound virus was washed away, and cells were immediately transferred to the microscope. Five z sections of each cell were imaged with a spinning-disc confocal microscope before (10 min after washing) and after (15 min after washing) the addition of trypan blue to quench extracellular fluorescence. (B) Same as A, but cells were incubated at 37°C for 240 min before quenching (at 244 min). (C) Same as A, but cells were preincubated with Baf 1 h before and during incubation with the virus for 240 min at 37°C.
Fig. 6.
SV40 trafficking is inhibited by an elevation of the vacuolar pH (see also Movies S5 and S6 in the supplemental material). (A, C, and D) SV40-AF647 was incubated with cells transfected with XFP-tagged proteins, and 100 nM bafilomycin A1 (Baf), 20 mM NH4Cl, or 10 μM monensin was added; cells were imaged by confocal microscopy at different time points postwarming. Note that NH4Cl and monensin cause a swelling of endosomes and lysosomes, and Baf leads to a loss of the round appearance of endosomes. (B) Percentage of viruses colocalizing with the markers Rab7-EGFP and Rab5-mRFP at different time points postwarming in the presence of 100 nM Baf, calculated from images such as those shown in A. Error bars are SEM of data from each time point for 5 to 15 cells from three independent experiments, with an average of 50 to 100 particles per cell.
Fig. 7.
Molecular requirements for SV40 passage through the late endosomal pathway and to the ER. (A) Percentage of SV40 particles internalized in the presence of 5 μM nocodazole (Noc) compared to mock-treated controls. (B and C) EM images of SV40 particles added to CV-1 cells in the presence of 5 μM nocodazole for 4 h. Viruses are indicated by black arrows. (D) SV40-AF647 was incubated with CV-1 cells transfected with Rab7-mRFP and Rab5-EGFP in the continuous presence of 5 μM nocodazole. A confocal slice of a representative cell imaged after 230 min is shown. (E) Percentage of virus particles colocalizing with Rab7-mRFP and Rab5-EGFP at different time points postwarming in the presence of 5 μM nocodazole, as quantified from images such as those shown in D. Error bars are SEM of data from each time point for 5 to 15 cells from three different experiments, with an average of 50 to 100 particles per cell. (F) CV-1 cells incubated with 5 μM nocodazole were infected with SV40 at an MOI of 5 for 24 h. The drug was washed out at 8 h postwarming, or different drugs were added after the washout. The infection level was determined by the immunostaining of the SV40 T antigen and flow cytometry normalized to drug-free controls. Shown are means ± SEM of data from three independent experiments, each with triplicate samples. Abbreviations: Baf, bafilomycin A1; Mon, monensin; BFA, brefeldin A; OV, orthovanadate; Gen, genistein; OA, okadaic acid; CalC, calphostin C; Dyn, dynasore. (G) Percentage of SV40 particles internalized in the presence of 80 μM dynasore compared to mock-treated controls.
Fig. 8.
Model of infectious SV40 entry into CV-1 cells. SV40 binds to its receptor (GM1), partitions into lipid rafts, and induces internalization from the plasma membrane either by a caveola-mediated or a caveolin-1-independent, lipid raft-dependent endocytosis mechanism. The virus is transported to Rab5-, EEA1-, and Hrs-positive EEs. When these endosomes acquire Rab7, SV40 associates with the Rab7-positive domains. Through endosome maturation, viruses become lumenal components of LAMP1-, Rab9-, and Rab7-positive LEs and eventually endolysosomes. The vacuolar ATPase (v-ATPase) is responsible for the acidification of endosomes and lysosomes. Acidification is required for SV40 internalization and subsequent transport steps. Virus transport to the ER occurs from the late compartments of the endocytic pathway by an unknown mechanism either directly or, less likely, via the Golgi complex. Early and late events in the entry pathway can be blocked by various inhibitors and other perturbants. References to the effects of different drugs reported in the literature are given.
Similar articles
- Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER.
Pelkmans L, Kartenbeck J, Helenius A. Pelkmans L, et al. Nat Cell Biol. 2001 May;3(5):473-83. doi: 10.1038/35074539. Nat Cell Biol. 2001. PMID: 11331875 - Echovirus 7 entry into polarized intestinal epithelial cells requires clathrin and Rab7.
Kim C, Bergelson JM. Kim C, et al. mBio. 2012 Apr 10;3(2):e00304-11. doi: 10.1128/mBio.00304-11. Print 2012. mBio. 2012. PMID: 22496312 Free PMC article. - Infectious bronchitis virus entry mainly depends on clathrin mediated endocytosis and requires classical endosomal/lysosomal system.
Wang H, Yuan X, Sun Y, Mao X, Meng C, Tan L, Song C, Qiu X, Ding C, Liao Y. Wang H, et al. Virology. 2019 Feb;528:118-136. doi: 10.1016/j.virol.2018.12.012. Epub 2018 Dec 28. Virology. 2019. PMID: 30597347 Free PMC article. - SV40 Hijacks Cellular Transport, Membrane Penetration, and Disassembly Machineries to Promote Infection.
Chen YJ, Liu X, Tsai B. Chen YJ, et al. Viruses. 2019 Oct 5;11(10):917. doi: 10.3390/v11100917. Viruses. 2019. PMID: 31590347 Free PMC article. Review. - [Caveolar endocytosis and virus entry].
Nomura R. Nomura R. Uirusu. 2005 Jun;55(1):19-26. doi: 10.2222/jsv.55.19. Uirusu. 2005. PMID: 16308526 Review. Japanese.
Cited by
- A nucleotide exchange factor promotes endoplasmic reticulum-to-cytosol membrane penetration of the nonenveloped virus simian virus 40.
Inoue T, Tsai B. Inoue T, et al. J Virol. 2015 Apr;89(8):4069-79. doi: 10.1128/JVI.03552-14. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653441 Free PMC article. - How Polyomaviruses Exploit the ERAD Machinery to Cause Infection.
Dupzyk A, Tsai B. Dupzyk A, et al. Viruses. 2016 Aug 29;8(9):242. doi: 10.3390/v8090242. Viruses. 2016. PMID: 27589785 Free PMC article. Review. - Non-viral Delivery of Nucleic Acids: Insight Into Mechanisms of Overcoming Intracellular Barriers.
Durymanov M, Reineke J. Durymanov M, et al. Front Pharmacol. 2018 Aug 21;9:971. doi: 10.3389/fphar.2018.00971. eCollection 2018. Front Pharmacol. 2018. PMID: 30186185 Free PMC article. Review. - Synchronized retrovirus fusion in cells expressing alternative receptor isoforms releases the viral core into distinct sub-cellular compartments.
Padilla-Parra S, Marin M, Kondo N, Melikyan GB. Padilla-Parra S, et al. PLoS Pathog. 2012;8(5):e1002694. doi: 10.1371/journal.ppat.1002694. Epub 2012 May 10. PLoS Pathog. 2012. PMID: 22589725 Free PMC article. - Inhibition of HIV-1 endocytosis allows lipid mixing at the plasma membrane, but not complete fusion.
de la Vega M, Marin M, Kondo N, Miyauchi K, Kim Y, Epand RF, Epand RM, Melikyan GB. de la Vega M, et al. Retrovirology. 2011 Dec 6;8:99. doi: 10.1186/1742-4690-8-99. Retrovirology. 2011. PMID: 22145853 Free PMC article.
References
- Baravalle G., et al. 2005. Transferrin recycling and dextran transport to lysosomes is differentially affected by bafilomycin, nocodazole, and low temperature. Cell Tissue Res. 320:99–113 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources