Hepcidin and iron regulation, 10 years later - PubMed (original) (raw)
Review
Hepcidin and iron regulation, 10 years later
Tomas Ganz. Blood. 2011.
Abstract
Under evolutionary pressure to counter the toxicity of iron and to maintain adequate iron supply for hemoglobin synthesis and essential metabolic functions, humans and other vertebrates have effective mechanisms to conserve iron and to regulate its concentration, storage, and distribution in tissues. The iron-regulatory hormone hepcidin, first described 10 years ago, and its receptor and iron channel ferroportin control the dietary absorption, storage, and tissue distribution of iron. Hepcidin causes ferroportin internalization and degradation, thereby decreasing iron transfer into blood plasma from the duodenum, from macrophages involved in recycling senescent erythrocytes, and from iron-storing hepatocytes. Hepcidin is feedback regulated by iron concentrations in plasma and the liver and by erythropoietic demand for iron. Genetic malfunctions affecting the hepcidin-ferroportin axis are a main cause of iron overload disorders but can also cause iron-restricted anemias. Modulation of hepcidin and ferroportin expression during infection and inflammation couples iron metabolism to host defense and decreases iron availability to invading pathogens. This response also restricts the iron supply to erythropoietic precursors and may cause or contribute to the anemia associated with infections and inflammatory disorders.
Figures
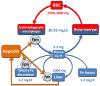
Figure 1
Hepcidin interaction with ferroportin controls the main iron flows into plasma. Iron flows and reservoirs are depicted in blue, iron in hemoglobin in red, and hepcidin and its effect in orange. RBC indicates red blood cell; and Fpn, ferroportin.
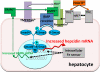
Figure 2
A current model of regulation of hepcidin transcription by iron. Iron as holotransferrin is shown in orange, iron sensors and associated molecule in gray, BMP receptor and its transduction pathway in shades of blue, the ligands and coreceptors of the BMP receptor in shades of green, and the negative regulator protease in purple. *Molecules whose ablation was shown to cause iron dysregulation.
Similar articles
- Hepcidin and iron homeostasis.
Ganz T, Nemeth E. Ganz T, et al. Biochim Biophys Acta. 2012 Sep;1823(9):1434-43. doi: 10.1016/j.bbamcr.2012.01.014. Epub 2012 Jan 26. Biochim Biophys Acta. 2012. PMID: 22306005 Free PMC article. Review. - Molecular control of iron transport.
Ganz T. Ganz T. J Am Soc Nephrol. 2007 Feb;18(2):394-400. doi: 10.1681/ASN.2006070802. Epub 2007 Jan 17. J Am Soc Nephrol. 2007. PMID: 17229910 Review. - Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis.
Nemeth E, Ganz T. Nemeth E, et al. Int J Mol Sci. 2021 Jun 17;22(12):6493. doi: 10.3390/ijms22126493. Int J Mol Sci. 2021. PMID: 34204327 Free PMC article. Review. - Hepcidin and ferroportin: the new players in iron metabolism.
De Domenico I, Ward DM, Kaplan J. De Domenico I, et al. Semin Liver Dis. 2011 Aug;31(3):272-9. doi: 10.1055/s-0031-1286058. Epub 2011 Sep 7. Semin Liver Dis. 2011. PMID: 21901657 Free PMC article. Review. - Hepcidin and disorders of iron metabolism.
Ganz T, Nemeth E. Ganz T, et al. Annu Rev Med. 2011;62:347-60. doi: 10.1146/annurev-med-050109-142444. Annu Rev Med. 2011. PMID: 20887198 Review.
Cited by
- Postencephalitic Parkinsonism: Unique Pathological and Clinical Features-Preliminary Data.
Strobel S, Sian-Hulsmann J, Tappe D, Jellinger K, Riederer P, Monoranu CM. Strobel S, et al. Cells. 2024 Sep 10;13(18):1511. doi: 10.3390/cells13181511. Cells. 2024. PMID: 39329695 Free PMC article. - Unraveling mechanisms regulating systemic iron homeostasis.
Finberg KE. Finberg KE. Hematology Am Soc Hematol Educ Program. 2011;2011:532-7. doi: 10.1182/asheducation-2011.1.532. Hematology Am Soc Hematol Educ Program. 2011. PMID: 22160085 Free PMC article. Review. - Modulation of iron metabolism in aging and in Alzheimer's disease: relevance of the choroid plexus.
Mesquita SD, Ferreira AC, Sousa JC, Santos NC, Correia-Neves M, Sousa N, Palha JA, Marques F. Mesquita SD, et al. Front Cell Neurosci. 2012 May 22;6:25. doi: 10.3389/fncel.2012.00025. eCollection 2012. Front Cell Neurosci. 2012. PMID: 22661928 Free PMC article. - Serum levels of the hepcidin-20 isoform in a large general population: the Val Borbera study.
Campostrini N, Traglia M, Martinelli N, Corbella M, Cocca M, Manna D, Castagna A, Masciullo C, Silvestri L, Olivieri O, Toniolo D, Camaschella C, Girelli D. Campostrini N, et al. J Proteomics. 2012 Dec 5;76 Spec No.(5):28-35. doi: 10.1016/j.jprot.2012.08.006. Epub 2012 Aug 21. J Proteomics. 2012. PMID: 22951294 Free PMC article. Clinical Trial. - Repressed Exercise-Induced Hepcidin Levels after Danggui Buxue Tang Supplementation in Male Recreational Runners.
Chang CW, Chen CY, Yen CC, Wu YT, Hsu MC. Chang CW, et al. Nutrients. 2018 Sep 18;10(9):1318. doi: 10.3390/nu10091318. Nutrients. 2018. PMID: 30231484 Free PMC article. Clinical Trial.
References
- Krause A, Neitz S, Magert HJ, et al. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480(2–3):147–150. - PubMed
- Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–7810. - PubMed
- Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276(11):7811–7819. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical