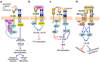Microbial manipulation of receptor crosstalk in innate immunity - PubMed (original) (raw)
Review
Microbial manipulation of receptor crosstalk in innate immunity
George Hajishengallis et al. Nat Rev Immunol. 2011 Mar.
Abstract
In the arms race of host-microbe co-evolution, successful microbial pathogens have evolved ingenious ways to evade host immune responses. In this Review, we focus on 'crosstalk manipulation' - the microbial strategies that instigate, subvert or disrupt the molecular signalling crosstalk between receptors of the innate immune system. This proactive interference undermines host defences and contributes to microbial adaptive fitness and persistent infections. Understanding how pathogens exploit host receptor crosstalk mechanisms and infiltrate the host signalling network is essential for developing interventions to redirect the host response and achieve protective immunity.
Figures
Figure 1. Inhibition of cell activation by pathogen-ligated ITIM-bearing or ITAMi-coupled receptors
a| Moraxella catarrhalis and Neisseria meningitidis use specific virulence proteins to activate carcinoembryonic antigen–related cell adhesion molecule 1 (CEACAM1), which co-associates with and inhibits Toll-like receptor-2 (TLR2) signalling. The underlying crosstalk involves phosphorylation of the CEACAM1 immunoreceptor tyrosine-based inhibitory motif (ITIM), which recruits Src homology 2 domain-containing protein-tyrosine phosphatase 1 (SHP1); this suppresses the phosphorylation of phosphatidylinositol-3 kinase (PI3K) and downstream activation of the AKT-mediated pro-inflammatory pathway. b| Serotypes of Group B Streptococcus (GBS) bind sialic acid-recognizing immunoglobulin-superfamily lectins (Siglecs), either through molecular mimicry of host sialylated glycans or through a cell wall-anchored protein. GBS activation of ITIM-bearing Siglec-5 or Siglec-9 activates inhibitory SHP2–dependent signals that interfere with cellular activation and antimicrobial functions. c| Staphylococcus aureus uses the ITIM-containing paired Ig-like receptor B (PIR-B) to crosstalk with and inhibit the TLR2-induced inflammatory response, possibly by inhibiting the PI3K–AKT pathway. d| Human cytomegalovirus (HCMV) expresses an MHC class I homologue, UL18, which interacts with ILT2 and activates ITIM-dependent and SHP1–mediated signalling. This inhibits natural killer (NK) cell activating receptors, such as the NK group 2, member C (NKG2C)–CD94 complex, and interferes with NK cell-mediated cytolysis of the HCMV-infected cell. e| Upon activation by viruses, the ITIM-bearing DC immunoreceptor (DCIR) becomes internalized into endosomes and inhibits endosomal TLRs — specifically, it inhibits production of TLR8-induced interleukin-12 (IL-12) and TLR9-induced interferon-α (IFNα) in conventional and plasmacytoid dendritic cells, respectively. f| Escherichia coli evades macrophage receptor with collagenous structure (MARCO)-dependent phagocytic killing through an inhibitory crosstalk with FcγRIII. Specifically, nonopsonized E. coli binds with low-affinity to FcγRIII and induces partial phosphorylation of the FcRγ ITAM (ITAMi), leading to weak mobilization of spleen tyrosine kinase (SYK) but strong recruitment of SHP1. SHP1 dephosphorylates PI3K and impairs MARCO-dependent phagocytosis.
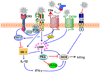
Figure 2. Pathogen-induced receptor crosstalk to stimulate IL-10 induction
a| The indicated pathogens express mannose-containing ligands that bind DC-specific intercellular adhesion molecule-grabbing non-integrin (DC-SIGN) and induce crosstalk with Toll-like receptors (TLRs) through RAF1 signalling. Induction of RAF1 signalling involves the participation of the LSP1–KSR1–CNK scaffolding complex and upstream activators (LARG, Ras, and RhoA) and mediates phosphorylation and acetylation of TLR-activated nuclear factor-κB (NF-κB) p65 subunit. This causes enhanced transcription of the Il10, Il12a and Il12b genes due to the enhanced DNA-binding affinity and transcriptional activity of acetylated p65. b| Helicobacter pylori binds DC-SIGN through fucose-containing lipopolysaccharide Lewis antigens and activates LSP1-dependent but RAF1-independent signalling, leading to increased IL-10, decreased IL-12 and the inhibition of TH1 cell development. c| Borrelia burgdorferi uses the salivary protein Salp15 of its tick vector to induce DC-SIGN–TLR crosstalk. Here, DC-SIGN-induced RAF1 signalling does not lead to p65 acetylation but stimulates MEK signalling, which promotes Il6 and Tnf mRNA decay and impairs nucleosome remodeling at the Il12a promoter. This divergent RAF1 pathway might be attributed to possible Salp15 binding to CD4, which might participate in the crosstalk. This DC-SIGN–TLR crosstalk does not destabilize Il10 mRNA, but, rather, IL-10 production is synergistically enhanced and leads to inhibition of DC maturation. d| In neutrophils, mycobacteria interact with a C-type lectin (possibly Clec5A) linked to immunoreceptor tyrosine-based activation motif (ITAM)-bearing DAP12. This interaction induces spleen tyrosine kinase (SYK)-dependent crosstalk with the TLR2–MYD88 (myeloid differentiation primary response protein 88) pathway, which synergistically upregulates IL-10 through sustained phosphorylation of AKT and p38 mitogen-activated protein kinase (MAPK). This decreases lung inflammation but increases the persistence of a high mycobacterial burden in a mouse lung infection model. CNK, connector enhancer of KSR; KSR1, kinase suppressor of Ras-1; LARG, leukemia-associated RhoA guanine-nucleotide-exchange factor; LSP1, leukocyte-specific protein-1.
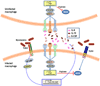
Figure 3. Integration of subversive crosstalk pathways leading to inhibition of pathogen killing
Porphyromonas gingivalis interacts with Toll-like receptor (TLR)-2 (specifically with the CD14–TLR2–TLR1 complex) and TLR4. The latter receptor is blocked by the bacterium’s atypical lipopolysaccharide (TLR4 antagonist) and thus cannot induce protective responses. The TLR2 response is proactively modified through crosstalk with other receptors that are regulated by P. gingivalis. P. gingivalis controls C5a receptor (C5aR) by virtue of Arg-specific cysteine proteinases, which attack C5 and release biologically active C5a. C5a stimulates intracellular Ca2+ signalling which synergistically enhances the otherwise weak cAMP responses induced by TLR2 activation alone. Maximal cAMP induction requires the participation of CXC-chemokine receptor 4 (CXCR4), which is activated directly by the pathogen’s fimbriae and coassociates with both TLR2 and C5aR in lipid rafts. The ensuing activation of the cAMP-dependent protein kinase A (PKA) inactivates glycogen synthase kinase-3β (GSK3β) and impairs the inducible nitric oxide synthase (iNOS)-dependent killing of the pathogen in macrophages. An additional pathway induced downstream of TLR2 is an inside-out signalling pathway, mediated by RAC1, phosphatidylinositol-3 kinase (PI3K) and cytohesin 1 (CYT1), which transactivates complement receptor-3 (CR3). Activated CR3 binds P. gingivalis and induces extracellular signal-regulated kinase-1 (ERK1)/ERK2 signalling, which in turn selectively downregulates IL-12 p35 and p40 mRNA expression through suppression of interferon regulatory factor 1 (IRF1). This inhibitory ERK1/ERK2 pathway is also activated downstream of the C5aR. Inhibition of bioactive IL-12, and secondarily IFNγ, leads to impaired immune clearance of P. gingivalis.
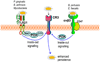
Figure 4. Pathogen-induced transactivation of CR3-mediated internalization
Certain bacteria (such as Porphyromonas gingivalis, Mycobacterium tuberculosis and Bacillus anthracis) bind CD14 and induce Toll-like receptor-2 (TLR2)–TLR1 inside-out signalling for activating and binding complement receptor-3 (CR3), which leads to a relatively ‘safe’ uptake of these organisms by macrophages. The signalling pathway that activates the high-affinity state of CR3 is mediated by RAC1, phosphatidylinositol-3 kinase (PI3K) and cytohesin 1 (CYT1). Enterococcus faecalis and Bordetella pertussis stimulate their uptake by CR3 through an alternative inside-out signalling pathway. This mechanism is activated by the interaction of these bacteria with a receptor complex comprising the αvβ3 integrin and CD47, and is dependent on PI3K signalling. Similarly, CR3-mediated uptake of these bacteria inhibits their intracellular killing and promotes their persistence in the mammalian host.
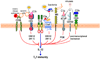
Figure 5. Selective inhibition of TLR-induced IL-12 production by pathogen-instigated PRR crosstalk
The crosstalk between anaphylatoxin receptors (particularly C5a receptor [C5aR]) or other complement receptors (such as complement receptor-3 [CR3], gC1q receptor [gC1qR] and CD46) and Toll-like receptors (TLRs) selectively inhibits the induction of IL-12. Relatively little is known regarding the pathways mediating this selective inhibition; signalling molecules that have been implicated, such as extracellular signal-regulated kinase-1 (ERK1) and ERK2 and phosphatidylinositol-3 kinase (PI3K), are shown downstream of the corresponding receptors. At least for ERK1 and ERK2, the selectivity of IL-12 inhibition is attributed to the suppression of a crucial transcription factor, the interferon regulatory factor 1 (IRF1). Posttranscriptional mechanisms might also contribute to IL-12 inhibition. Activation of these complement receptors, or other innate immune receptors (such as CD36, mannose receptor and CD150) that share the ability to downregulate IL-12, by their natural ligands might have a homeostatic function. However, these same receptors can be activated by bacterial, viral or parasitic pathogens, which can thereby downregulate TLR-induced IL-12 production to interfere with host defences (such as the inhibition of TH1 cell-mediated immunity). Although microbial molecules that act as ligands for C5aR have been described, this receptor can come under pathogen control also through the enzymatic generation of high levels of C5a by microbial C5 convertase-like enzymes.
Figure 6. MyD88-dependent arginase induction prevents nitric oxide production in both mycobacteria-infected and -uninfected macrophages
Activation of myeloid differentiation primary response protein 88 (MYD88) signalling by mycobacteria (at least in part through TLR2) induces CCAAT/enhancer-binding protein β (C/EBPβ)-mediated induction of interleukin-6 (IL-6), IL-10, and granulocyte colony-stimulating factor (GCSF) production. These signal transducer and activator of transcription-3 (STAT3)-activating cytokines act in both autocrine and paracrine manners to induce arginase 1 (Arg1) expression which is partially dependent on C/EBPβ. The produced arginase can inhibit inducible nitric oxide synthase (iNOS) activity through competition for the common substrate arginine. The MYD88 pathway for arginase production was shown to confer a survival benefit for mycobacteria in vivo and is thought to counteract pathways that activate nitric oxide production, such as TLR4.
References
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature Immunol. 2010;11:373–384. - PubMed
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nature Immunol. 2010;11:785–797. A comprehensive review of the complement system, summarizing recent and emerging evidence that complement engages in reciprocal crosstalk interactions with other immune and physiological systems (such as TLRs and coagulation), aiming to fine-tune the host response to infection and other insults.
- Medzhitov R. Toll-like receptors and innate immunity. Nature Rev. Immunol. 2001;1:135–145. - PubMed
- Triantafilou K, Triantafilou M, Dedrick RL. A CD14-independent LPS receptor cluster. Nature Immunol. 2001;2:338–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC4 DE021580/DE/NIDCR NIH HHS/United States
- R01 DE015254/DE/NIDCR NIH HHS/United States
- CA112162/CA/NCI NIH HHS/United States
- AI72106/AI/NIAID NIH HHS/United States
- DE018292/DE/NIDCR NIH HHS/United States
- DE021580/DE/NIDCR NIH HHS/United States
- GM62134/GM/NIGMS NIH HHS/United States
- R01 GM062134/GM/NIGMS NIH HHS/United States
- R01 EB003968/EB/NIBIB NIH HHS/United States
- N01 AI030040/AI/NIAID NIH HHS/United States
- R01 DE018292/DE/NIDCR NIH HHS/United States
- AI30040/AI/NIAID NIH HHS/United States
- DE017138/DE/NIDCR NIH HHS/United States
- R01 AI030040/AI/NIAID NIH HHS/United States
- DE015254/DE/NIDCR NIH HHS/United States
- AI68730/AI/NIAID NIH HHS/United States
- EB3968/EB/NIBIB NIH HHS/United States
- R01 CA112162/CA/NCI NIH HHS/United States
- R01 DE017138/DE/NIDCR NIH HHS/United States
- P01 AI068730/AI/NIAID NIH HHS/United States
- R01 AI072106/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources