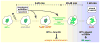Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair - PubMed (original) (raw)
Double-strand breaks in heterochromatin move outside of a dynamic HP1a domain to complete recombinational repair
Irene Chiolo et al. Cell. 2011.
Abstract
Double-strand breaks (DSBs) in heterochromatic repetitive DNAs pose significant threats to genome integrity, but information about how such lesions are processed and repaired is sparse. We observe dramatic expansion and dynamic protrusions of the heterochromatin domain in response to ionizing radiation (IR) in Drosophila cells. We also find that heterochromatic DSBs are repaired by homologous recombination (HR) but with striking differences from euchromatin. Proteins involved in early HR events (resection) are rapidly recruited to DSBs within heterochromatin. In contrast, Rad51, which mediates strand invasion, only associates with DSBs that relocalize outside of the domain. Heterochromatin expansion and relocalization of foci require checkpoint and resection proteins. Finally, the Smc5/6 complex is enriched in heterochromatin and is required to exclude Rad51 from the domain and prevent abnormal recombination. We propose that the spatial and temporal control of DSB repair in heterochromatin safeguards genome stability by preventing aberrant exchanges between repeats.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
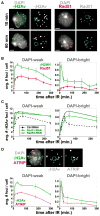
Figure 1. Processing of DSBs in Heterochromatin Occurs with Different Kinetics Than Euchromatin and Requires HR
(A) γH2Av, but not Rad51, foci form in DAPI-bright (dashed circles) after IR. IF with γH2Av or Rad51 antibodies shows γH2Av foci in DAPI-bright at 10 min and not at 60 min after IR. Rad51 foci are rare in DAPI-bright at both time points (see one example in Figure S2B). (B) IF was performed at different time points after IR as in (A), and quantitative analysis of foci was performed. In DAPI-weak, frequencies of γH2Av and Rad51 foci peak at 30 and 60 min after IR, respectively, and then show a slow decay. In DAPI-bright, rapid reduction of γH2Av foci is observed between 10 and 30 min after IR (p < 0.001; n > 60). No significant differences in the low numbers of Rad51 foci were observed in DAPI-bright throughout the time course (n > 100). (C) HR proteins are required for removal of γH2Av foci from heterochromatin. Quantitative kinetic analysis shows persistent γH2Av foci in DAPI-bright and DAPI-weak after Rad51 or Rad54 RNAi at 60 and 240 min after IR (p < 0.01; n > 100). Examples of cells are shown in Figure S2E. (D) ATRIP foci form in DAPI-bright in response to IR. Cells expressing GFP-ATRIP were fixed 10 min after IR and stained with γH2Av antibodies (top). Quantitation (bottom) reveals rapid reduction of ATRIP and γH2Av foci between 10 and 30 min after IR (p = 0.001; n > 60). In DAPI-weak, the kinetics of ATRIP foci is similar to Rad51 (see B). Graphs show mean ± SEM. Only Z-stacks including the DAPI-bright are shown as projections in (A) and (D). Scale bars, 1 μm. See also Figure S1 and Figure S2.
Figure 2. Heterochromatin Expands and Becomes Dynamic in Response to IR
(A) HP1a expands after IR and forms dynamic protrusions. Stills from Movie S1 are shown, in which a single cell expressing GFP-HP1a was imaged before IR (−IR) and starting 20 min after IR (+IR). Arrows and zoomed detail of the outlined region show HP1a protrusions. Scale bars, 5 μm. (B) Automated quantitation shows HP1a expansion (p < 0.0001) followed by partial contraction (p = 0.0011) (see examples in Figure S3B). After contraction, the volume of HP1a is still significantly larger than the untreated control (p < 0.0001) (two-tailed unpaired t test; n = 11; graph shows mean ± SEM). (C) ChIP-array analysis of HP1a localization was performed before (UNT), 30 min, and 60 min after IR. The browser view of the proximal half of chromosome 3L shows the position of the euchromatin-heterochromatin border (red line); x axis = arm coordinates in Mbs. y axis = enrichment p values, normalized to input. No major increases in HP1a enrichments are observed in the euchromatin after IR (other chromosome arms are shown in Figure S3). (D) IR induces expansion of heterochromatin sequences. Quantitation of the distance between AACAC signals, detected by FISH, and DAPI-bright shows higher values between 10 and 60 min after IR and partial decrease at 180 min (p < 0.001) (n = 150 signals). Red bar, mean; black bars, ± SEM. See also Figure S3 and Movie S1.
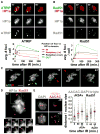
Figure 3. Heterochromatic DSBs Relocalize Outside of the HP1a Domain, Where They Associate with Rad51
(A) ATRIP foci form within the HP1a domain and relocate outside of the domain. Stills from Movie S2 are shown, in which cells expressing GFP-ATRIP and mCh-HP1a were imaged before and after IR. (Top) Maximum intensity projections of one cell at 10, 30, and 60 min after IR show the relocalization of ATRIP foci to the periphery and outside of the HP1a domain. More time points are shown in Figure S4C. (Bottom) Quantitation shows increased ATRIP foci within the HP1a domain at 4 and 10 min and reductions after 10 min. ATRIP foci accumulate at the periphery of the HP1a domain from 10 to 60 min (p < 0.001; n = 30 cells). (B) Rad51 foci form at the periphery and outside of the HP1a domain after IR. Cells expressing GFP-Rad51 and mCh-HP1a were analyzed as in (A). (Top) Images of the same cell at 10, 30, and 60 min after IR; more time points are shown in Figure S4E. (Bottom) Quantitation shows that Rad51 foci only appear near the HP1a periphery or outside of the domain (n = 40 cells). (C) 3D modeling of stills from Movie S3 (Figure S5A), in which cells expressing GFP-ATRIP and mCh-HP1a were imaged before and after IR, shows ATRIP foci formed within the HP1a domain that translocate outside (arrows) and frequently split (see red, cyan, yellow foci). (D) Cells expressing GFP-Rad51 and mCh-HP1a, imaged before and after IR, show Rad51 foci localized at the “tips” of dynamic HP1a protrusions. Stills from Movie S5 are shown. Representative time points of the region outlined are shown below. (E) Damaged heterochromatic repeats are more distant from DAPI-bright compared to undamaged repeats. AACAC repeats and γH2Av or Rad51 foci were detected by FISH-IF of cells fixed before (−) and 60 min after (+) IR. Images and quantitation of the distance from DAPI-bright (dashed blue circle) show that AACAC signals that colocalize with γH2Av (60 + γH2Av) or Rad51 (60 + Rad51) are more distant than all AACAC signals at the same time point (60) (p < 0.001 and p < 0.02, respectively, by two-tailed Mann-Whitney test; n > 100 signals). The distances in untreated cells are also shown for each experiment (0). Images are projections of the Z-stacks, including the DAPI-bright. Graphs show mean ± SEM. Scale bars, 1 μm. See also Figure S4, Figure S5, Movie S2, Movie S3, Movie S4, and Movie S5.
Figure 4. Checkpoint Kinases and Resection Are Required for Heterochromatin Expansion and DSB Relocalization
(A) Caffeine treatment suppresses HP1a dynamics and prevents ATRIP foci assembly within the HP1a domain. Stills from Movie S6 are shown, in which cells expressing GFP-ATRIP and mCh-HP1a were imaged before and after IR, in the presence (+ caffeine) or absence (Ctrl) of caffeine. Scale bar, 1 μm. (B) Quantitation of the experiment described in (A) shows defective expansion of the HP1a volume after caffeine treatment compared to untreated cells (p < 0.0001; n > 15). (C) Quantitation of the experiment described in (A) highlights defective formation of ATRIP foci in the HP1a domain after caffeine treatment (p < 0.001; n > 30). ATM is not required for formation of ATRIP foci in heterochromatin (Figure S6B), suggesting that this defect results from ATR inactivation. (D) Quantitation of HP1a volume shows defective expansion after depletion of checkpoint or resection proteins. Values = mean fold increase in HP1a volume for time points between 10 and 40 min after IR, compared to control RNAi (***p < 0.0001, **p < 0.01, two-tailed unpaired t test with Welch correction; n > 12). All time points are shown in Figure S6E. (E) Depletion of checkpoint or resection proteins results in persistent γH2Av foci in DAPI-bright at 60 min after IR (***p < 0.0001, **p < 0.01, *p < 0.05, two-tailed Mann-Whitney test; n > 100). Kinetics are shown in Figure S6F. (F) RNAi of CtIP+Tosca+Blm results in persistent Mu2 foci within the HP1a domain after IR and defective relocalization to the HP1a periphery (p < 0.0001; n > 20). This analysis could not be performed after ATR depletion because Mu2 foci do not form (Figure S6B). Similarly, ATRIP foci could not be monitored after RNAi of ATR or resection components (Figures S6B–S6D). Graphs show mean ± SEM. See also Figure S6 and Movie S6.
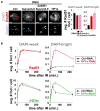
Figure 5. HP1a or H3K9 HMTase Depletion Results in Formation of Rad51 Foci in Heterochromatin
(A) IF analysis 60 min after IR and quantitation shows that Su(var)3–9, Su(var)3–9 + SetDB1, or HP1a depletion (quantitation is in B) results in increased Rad51 foci in DAPI-bright (dashed blue circles) and not in DAPI-weak (3- to 4-fold increase in DAPI-bright after Su(var)3–9 and SetDB1 RNAi [right]; p < 0.001; n > 100). (B) Quantitation of γH2Av and Rad51 foci at different time points after IR shows that depletion of HP1a results in increased foci in DAPI-bright (*p < 0.01; n > 100) and not in DAPI-weak. Graphs show mean ± SEM. Scale bars, 1 μm. See also Figure S7.
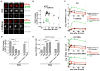
Figure 6. The Smc5/6 Complex Is Required to Prevent IR-Induced Rad51 Foci and Aberrant Recombination in Heterochromatin
(A) Images of cells expressing GFP-tagged Smc5, Smc6, or Nse2 and mCh-HP1a show that different Smc5/6 subunits colocalize with HP1a. (B) The Smc5/6 complex interacts with HP1a. FLAG-HP1a (+) expressing S2 cells were used to immunoprecipitate HP1a. Western blot with anti-FLAG (HP1a), Smc5, and Smc6 antibodies shows enrichment of Smc5 and Smc6 compared to control cells (−). Extracts were treated with Benzonase prior to IP, suggesting that Smc5/6-HP1a interaction is not mediated by DNA. (C) HP1a is required for localization of the Smc5/6 complex to heterochromatin. HP1a RNAi results in delocalization of GFP-Nse2 from heterochromatin (see Figure S8B for quantitation). (D) Smc5/6 and HP1a are part of the same pathway that excludes Rad51 foci from heterochromatin. Bw (control), HP1a, Smc5/6, or HP1a + Smc5/6 were depleted for 5 days by RNAi, and then cells were fixed after IR. Quantitation shows similar increases in DAPI-bright Rad51 foci after HP1a or Smc5/6 + HP1a RNAi (n > 150). Graphs show mean ± SEM. (E) Smc5/6 depletion alone (without IR) results in heterochromatic DNA filaments connecting dividing cells. Smc5 and Smc6 were depleted for 6.5 days by RNAi, spun down, mixed, and settled to adhere to the slide. Then, cells were fixed and stained with DAPI and H3K9me2 antibodies. Images are maximum intensity projections. The brightness of the DAPI-only image was uniformly increased to visualize the filaments. Similar results were observed after separate depletion of Smc5 or Smc6 (Figure S8E). (F) DNA filaments formed in the absence of Smc5/6 are dependent on recombination. Bw (control), Smc5/6, Rad51, and/or Rad54 were depleted and cells were processed as in (E). DNA filaments resulting from Smc5/6 RNAi are suppressed by simultaneous depletion of Rad51 and/or Rad54 (p < 0.0001; n > 500). Scale bars, 5 μm. See also Figure S8.
Figure 7. A Model for the Spatiotemporal Regulation of DSB Repair Events in Heterochromatin
DSB detection, checkpoint activation, and resection occur quickly in heterochromatin, where ATR and HP1a facilitate the assembly of TopBP1/ATRIP foci. Meanwhile, Rad51 recruitment and strand invasion are suppressed by the Smc5/6 complex, which is recruited to heterochromatin by HP1a (yellow background). Heterochromatin expansion facilitates DSB relocalization to the HP1a periphery; both dynamic behaviors require checkpoint kinases (mainly ATR) and resection proteins. The euchromatic environment or local disassembly of HP1a at DSBs allows Rad51 loading and completion of HR repair (blue background). Once initiated, strand invasion facilitates the retention of DSBs at the HP1a periphery or the euchromatic domain during HP1a contraction. This mechanism prevents ectopic recombination by isolating the damaged site from the undamaged heterochromatic repeats before strand invasion. Homolog or chromatid pairing, maintained during relocalization, likely provide the templates for completing HR repair. Only processes related to DSB repair in heterochromatin are shown. See Movie S7 for animated version.
Comment in
- The ins and outs of heterochromatic DNA repair.
Peterson CL. Peterson CL. Dev Cell. 2011 Mar 15;20(3):285-7. doi: 10.1016/j.devcel.2011.02.009. Dev Cell. 2011. PMID: 21397838 Free PMC article.
Similar articles
- Double-strand breaks in facultative heterochromatin require specific movements and chromatin changes for efficient repair.
Wensveen MR, Dixit AA, van Schendel R, Kendek A, Lambooij JP, Tijsterman M, Colmenares SU, Janssen A. Wensveen MR, et al. Nat Commun. 2024 Oct 17;15(1):8984. doi: 10.1038/s41467-024-53313-2. Nat Commun. 2024. PMID: 39419979 Free PMC article. - Timely double-strand break repair and pathway choice in pericentromeric heterochromatin depend on the histone demethylase dKDM4A.
Janssen A, Colmenares SU, Lee T, Karpen GH. Janssen A, et al. Genes Dev. 2019 Jan 1;33(1-2):103-115. doi: 10.1101/gad.317537.118. Epub 2018 Dec 21. Genes Dev. 2019. PMID: 30578303 Free PMC article. - A single double-strand break system reveals repair dynamics and mechanisms in heterochromatin and euchromatin.
Janssen A, Breuer GA, Brinkman EK, van der Meulen AI, Borden SV, van Steensel B, Bindra RS, LaRocque JR, Karpen GH. Janssen A, et al. Genes Dev. 2016 Jul 15;30(14):1645-57. doi: 10.1101/gad.283028.116. Genes Dev. 2016. PMID: 27474442 Free PMC article. - Nuclear Dynamics of Heterochromatin Repair.
Amaral N, Ryu T, Li X, Chiolo I. Amaral N, et al. Trends Genet. 2017 Feb;33(2):86-100. doi: 10.1016/j.tig.2016.12.004. Epub 2017 Jan 16. Trends Genet. 2017. PMID: 28104289 Free PMC article. Review. - The influence of heterochromatin on DNA double strand break repair: Getting the strong, silent type to relax.
Goodarzi AA, Jeggo P, Lobrich M. Goodarzi AA, et al. DNA Repair (Amst). 2010 Dec 10;9(12):1273-82. doi: 10.1016/j.dnarep.2010.09.013. Epub 2010 Oct 30. DNA Repair (Amst). 2010. PMID: 21036673 Review.
Cited by
- Cockayne syndrome group B protein regulates DNA double-strand break repair and checkpoint activation.
Batenburg NL, Thompson EL, Hendrickson EA, Zhu XD. Batenburg NL, et al. EMBO J. 2015 May 12;34(10):1399-416. doi: 10.15252/embj.201490041. Epub 2015 Mar 27. EMBO J. 2015. PMID: 25820262 Free PMC article. - Structural Insights into Ring Formation of Cohesin and Related Smc Complexes.
Gligoris T, Löwe J. Gligoris T, et al. Trends Cell Biol. 2016 Sep;26(9):680-693. doi: 10.1016/j.tcb.2016.04.002. Epub 2016 Apr 28. Trends Cell Biol. 2016. PMID: 27134029 Free PMC article. Review. - Epigenetic regulation of genomic integrity.
Deem AK, Li X, Tyler JK. Deem AK, et al. Chromosoma. 2012 Apr;121(2):131-51. doi: 10.1007/s00412-011-0358-1. Epub 2012 Jan 17. Chromosoma. 2012. PMID: 22249206 Free PMC article. Review. - Opportunities and challenges of radiotherapy for treating cancer.
Schaue D, McBride WH. Schaue D, et al. Nat Rev Clin Oncol. 2015 Sep;12(9):527-40. doi: 10.1038/nrclinonc.2015.120. Epub 2015 Jun 30. Nat Rev Clin Oncol. 2015. PMID: 26122185 Free PMC article. Review. - The heterochromatic barrier to DNA double strand break repair: how to get the entry visa.
Goodarzi AA, Jeggo PA. Goodarzi AA, et al. Int J Mol Sci. 2012;13(9):11844-11860. doi: 10.3390/ijms130911844. Epub 2012 Sep 19. Int J Mol Sci. 2012. PMID: 23109886 Free PMC article. Review.
References
- Ayoub N, Jeyasekharan AD, Bernal JA, Venkitaraman AR. HP1-beta mobilization promotes chromatin changes that initiate the DNA damage response. Nature. 2008;453:682–686. - PubMed
- Baumann C, Körner R, Hofmann K, Nigg EA. PICH, a centromere-associated SNF2 family ATPase, is regulated by Plk1 and required for the spindle checkpoint. Cell. 2007;128:101–114. - PubMed
- Bernard P, Maure JF, Partridge JF, Genier S, Javerzat JP, Allshire RC. Requirement of heterochromatin for cohesion at centromeres. Science. 2001;294:2539–2542. - PubMed
- Branzei D, Foiani M. Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol. 2010;11:208–219. - PubMed
- Chuang C-H, Carpenter AE, Fuchsova B, Johnson T, de Lanerolle P, Belmont AS. Long-range directional movement of an interphase chromosome site. Curr Biol. 2006;16:825–831. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous