Mechanism of membrane curvature sensing by amphipathic helix containing proteins - PubMed (original) (raw)
Mechanism of membrane curvature sensing by amphipathic helix containing proteins
Haosheng Cui et al. Biophys J. 2011.
Abstract
There are several examples of membrane-associated protein domains that target curved membranes. This behavior is believed to have functional significance in a number of essential pathways, such as clathrin-mediated endocytosis, which involve dramatic membrane remodeling and require the recruitment of various cofactors at different stages of the process. This work is motivated in part by recent experiments that demonstrated that the amphipathic N-terminal helix of endophilin (H0) targets curved membranes by binding to hydrophobic lipid bilayer packing defects which increase in number with increasing membrane curvature. Here we use state-of-the-art atomistic simulation to explore the packing defect structure of curved membranes, and the effect of this structure on the folding of H0. We find that not only are packing defects increased in number with increasing membrane curvature, but also that their size distribution depends nontrivially on the curvature, falling off exponentially with a decay constant that depends on the curvature, and crucially that even on highly curved membranes defects large enough to accommodate the hydrophobic face of H0 are never observed. We furthermore find that a percolation model for the defects explains the defect size distribution, which implies that larger defects are formed by coalescence of noninteracting smaller defects. We also use the recently developed metadynamics algorithm to study in detail the effect of such defects on H0 folding. It is found that the comparatively larger defects found on a convex membrane promote H0 folding by several kcal/mol, while the smaller defects found on flat and concave membrane surfaces inhibit folding by kinetically trapping the peptide. Together, these observations suggest H0 folding is a cooperative process in which the folding peptide changes the defect structure relative to an unperturbed membrane.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
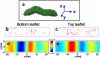
Figure 1
(a) A configuration of the protein-free membrane, with a region of high curvature imposed by the boundary conditions. At the region of highest curvature, the membrane has a radius of curvature of roughly 11 nm. (b) Red regions indicate packing defects in the bottom leaf of the membrane patch at one instant, mapped as described in Methods. (c) Packing defect structure of the top leaflet. (d) Curvature of the bottom leaflet, indicated by the color scale bar between panels d and e. (e) Curvature of the top leaflet.
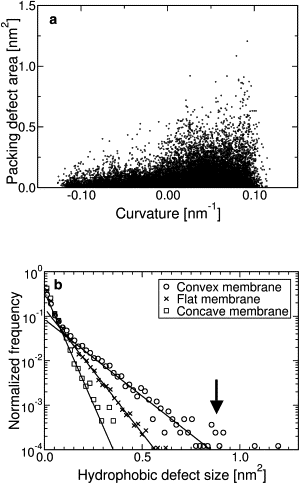
Figure 2
(a) Scatter plot showing relation between defect area and local curvature of the membrane. Each point is a single defect. (b) Histogram of defect areas for convex, flat, and concave membranes (symbols). Solid lines are least-squares fits to an exponential decay, decay constants in units of nm−2 are 7.3 (convex membrane), 12.8 (flat membrane), and 18.9 (concave membrane).
Figure 3
Two-dimensional potential of mean force for folding H0 in bulk solvent. Each locally stable basin is illustrated by a ribbon representation image of a typical configuration. The most stable state is the center of the three basins, thermodynamically favored by 2 kcal/mol over the _α_-helical state. (The peptides are color-coded throughout the article as follows: red, negatively charged residue; blue, positively charged residue; green, polar residue; white, hydrophobic residue.)
Figure 4
Two-dimensional potential of mean force for folding H0 at a convex membrane surface. The significantly larger packing defects found at a convex membrane surface promote folding of H0—now the _α_-helical state is favored over the unfolded states by 3 kcal/mol. The peptide color-coding is explained in the caption to Fig. 3.
Figure 5
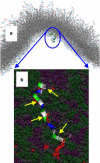
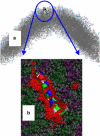
Example of a kinetically trapped configuration at a convex membrane surface. (a) Position of H0 from afar; note its position near the region of maximum concave curvature. (b) Detail of how H0 binds membranes presenting mostly small defects. (The hydrocarbon chains of the lipids are rendered in space-filling representation: gray, hydrocarbon chains; purple, PS headgroups; green, PC headgroups.) Portions of the hydrocarbon chains that would be exposed to solvent if not for the peptide (small red spheres), calculated as described in Supporting Methods in the Supporting Material. (Yellow arrows) Hydrophobic residues in contact with packing defects.
Figure 6
Example of a kinetically trapped configuration at a flat membrane surface. (a) Position of H0 from afar, note its position at a region of near-zero curvature. (b) A detail of the peptide binding is shown; note the larger size of the packing defects compared to the concave membrane. See Fig. 5 legend for an explanation of the rendering.
Figure 7
Example of H0 stably folded at a convex membrane surface. (a) Position of H0 near the region of maximum convex curvature. (b) A detail of the membrane binding of H0 on a large defect encountered at a convex membrane surface. Note the large hydrophobic surface that is in contact with the peptide, sufficient to accommodate the hydrophobic face of H0 when folded. Rendering colors explained in the caption to Fig. 5.
Similar articles
- The N-Terminal Amphipathic Helix of Endophilin Does Not Contribute to Its Molecular Curvature Generation Capacity.
Chen Z, Zhu C, Kuo CJ, Robustelli J, Baumgart T. Chen Z, et al. J Am Chem Soc. 2016 Nov 9;138(44):14616-14622. doi: 10.1021/jacs.6b06820. Epub 2016 Oct 28. J Am Chem Soc. 2016. PMID: 27755867 Free PMC article. - Charge distribution and helicity tune the binding of septin's amphipathic helix domain to membranes.
Edelmaier CJ, Klawa SJ, Mofidi SM, Wang Q, Bhonge S, Vogt EJD, Curtis BN, Shi W, Hanson SM, Klotsa D, Forest MG, Gladfelter AS, Freeman R, Nazockdast E. Edelmaier CJ, et al. Biophys J. 2025 Apr 15;124(8):1298-1312. doi: 10.1016/j.bpj.2025.03.008. Epub 2025 Apr 2. Biophys J. 2025. PMID: 40179880 - Amphipathic lipid packing sensor motifs: probing bilayer defects with hydrophobic residues.
Vanni S, Vamparys L, Gautier R, Drin G, Etchebest C, Fuchs PF, Antonny B. Vanni S, et al. Biophys J. 2013 Feb 5;104(3):575-84. doi: 10.1016/j.bpj.2012.11.3837. Biophys J. 2013. PMID: 23442908 Free PMC article. - Amphipathic helices and membrane curvature.
Drin G, Antonny B. Drin G, et al. FEBS Lett. 2010 May 3;584(9):1840-7. doi: 10.1016/j.febslet.2009.10.022. Epub 2009 Oct 20. FEBS Lett. 2010. PMID: 19837069 Review. - The Many Faces of Amphipathic Helices.
Giménez-Andrés M, Čopič A, Antonny B. Giménez-Andrés M, et al. Biomolecules. 2018 Jul 5;8(3):45. doi: 10.3390/biom8030045. Biomolecules. 2018. PMID: 29976879 Free PMC article. Review.
Cited by
- The N-Terminal Amphipathic Helix of Endophilin Does Not Contribute to Its Molecular Curvature Generation Capacity.
Chen Z, Zhu C, Kuo CJ, Robustelli J, Baumgart T. Chen Z, et al. J Am Chem Soc. 2016 Nov 9;138(44):14616-14622. doi: 10.1021/jacs.6b06820. Epub 2016 Oct 28. J Am Chem Soc. 2016. PMID: 27755867 Free PMC article. - The role of lipids in α-synuclein misfolding and neurotoxicity.
Ugalde CL, Lawson VA, Finkelstein DI, Hill AF. Ugalde CL, et al. J Biol Chem. 2019 Jun 7;294(23):9016-9028. doi: 10.1074/jbc.REV119.007500. Epub 2019 May 7. J Biol Chem. 2019. PMID: 31064841 Free PMC article. Review. - Membrane-binding peptides for extracellular vesicles on-chip analysis.
Gori A, Romanato A, Greta B, Strada A, Gagni P, Frigerio R, Brambilla D, Vago R, Galbiati S, Picciolini S, Bedoni M, Daaboul GG, Chiari M, Cretich M. Gori A, et al. J Extracell Vesicles. 2020 Apr 17;9(1):1751428. doi: 10.1080/20013078.2020.1751428. eCollection 2020. J Extracell Vesicles. 2020. PMID: 32363015 Free PMC article. - Multiscale computational models in physical systems biology of intracellular trafficking.
Tourdot RW, Bradley RP, Ramakrishnan N, Radhakrishnan R. Tourdot RW, et al. IET Syst Biol. 2014 Oct;8(5):198-213. doi: 10.1049/iet-syb.2013.0057. IET Syst Biol. 2014. PMID: 25257021 Free PMC article. - Membrane Remodeling Driven by Shallow Helix Insertions via a Cooperative Mechanism.
Hu J, Fu Y. Hu J, et al. Membranes (Basel). 2025 Apr 1;15(4):101. doi: 10.3390/membranes15040101. Membranes (Basel). 2025. PMID: 40277971 Free PMC article.
References
- Slepnev V.I., De Camilli P. Accessory factors in clathrin-dependent synaptic vesicle endocytosis. Nat. Rev. Neurosci. 2000;1:161–172. - PubMed
- McMahon H.T., Gallop J.L. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. - PubMed
- Zimmerberg J., Kozlov M.M. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 2006;7:9–19. - PubMed
- Doherty G.J., McMahon H.T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009;78:857–902. - PubMed
- Hatzakis N.S., Bhatia V.K., Stamou D. How curved membranes recruit amphipathic helices and protein anchoring motifs. Nat. Chem. Biol. 2009;5:835–841. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources