Snapin mediates incretin action and augments glucose-dependent insulin secretion - PubMed (original) (raw)
Snapin mediates incretin action and augments glucose-dependent insulin secretion
Woo-Jin Song et al. Cell Metab. 2011.
Abstract
Impaired insulin secretion contributes to the pathogenesis of type 2 diabetes mellitus (T2DM). Treatment with the incretin hormone glucagon-like peptide-1 (GLP-1) potentiates insulin secretion and improves metabolic control in humans with T2DM. GLP-1 receptor-mediated signaling leading to insulin secretion occurs via cyclic AMP stimulated protein kinase A (PKA)- as well as guanine nucleotide exchange factor-mediated pathways. However, how these two pathways integrate and coordinate insulin secretion remains poorly understood. Here we show that these incretin-stimulated pathways converge at the level of snapin, and that PKA-dependent phosphorylation of snapin increases interaction among insulin secretory vesicle-associated proteins, thereby potentiating glucose-stimulated insulin secretion (GSIS). In diabetic islets with impaired GSIS, snapin phosphorylation is reduced, and expression of a snapin mutant, which mimics site-specific phosphorylation, restores GSIS. Thus, snapin is a critical node in GSIS regulation and provides a potential therapeutic target to improve β cell function in T2DM.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Figure 1. Prkar1a ablation in pancreatic islets
Immunoblot (left) with densitometric analysis (right) of total islet protein from wt-prkar1a, het-prkar1a, Δ-prkar1a mice. Specific Prkar1a ablation is detectable, while other prkar subtypes and Pkac remain unchanged. Prkar1a expression is approximately 50% reduced in het-prkar1a islets and 90% reduced in Δ-prkar1a islets. CREB phosphorylation increases with reduced Prkar1a abundance.
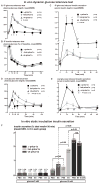
Figure 2. Glucose stimulated insulin secretion in Δ-prkar1a, het-prkar1a, and wt-prkar1a mice in vivo and from respective mouse islets in vitro
A) Plasma glucose levels during an ipGTT in littermates of indicated genotypes. Δ-prkar1a mice have markedly diminished glucose excursion during ipGTT but do not exhibit baseline or post glucose hypoglycemia. (* p<0.05). B) Plasma glucose levels during ipITT in littermates of indicated genotypes. No difference is seen in insulin sensitivity among the different genotypes. (* p<0.05). C) Serum insulin levels during ipGTT in littermates of indicated genotypes. All animals have similar baseline insulin levels. Δ-prkar1a mice have markedly increased glucose stimulated insulin levels, predominantly during the initial phases of ipGTT (* p<0.05). D) Plasma glucose levels during an oGTT in littermates of indicated genotypes. Baseline glucose levels are similar in all animals. Δ-prkar1a and het-prkar1a mice, respectively, have marked and graded reductions in glucose excursion during oGTT. Neither exhibit baseline or post glucose hypoglycemia. (*, # p<0.05 vs wt). E) Serum insulin levels during oGTT in littermates of indicated genotypes. All animals have similar baseline insulin levels. Δ-prkar1a mice have marked and graded increases in glucose stimulated insulin levels (*, # p<0.05 vs wt). F) In vitro static insulin secretion assay of islets cultured in glucose (3 and 10 mM) without and with E4 (10 nM) stimulated insulin secretion (* p<0.05). Accumulated insulin in supernatant was normalized to insulin in corresponding islets and provided in % islet insulin content
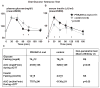
Figure 3. Oral GTT after in humans with inactivating PRKAR1A mutations and controls
A) Subjects with a PRKAR1A mutation exhibit normal fasting glucose levels but reduced glucose excursion after an oral glucose load. B) In subjects with a PRKAR1A mutation serum insulin levels were not different at baseline, and reached a higher peak with increased overall insulin secretion. C) Table summarizing fasting glucose and insulin levels as well as area under the glucose and insulin curves shown in A and B (*p<0.05)
Figure 4. Snapin is present in insulinoma cells and in mouse and human islets. E4 stimulates snapin serine-phosphorylation in a PKA-dependent manner
A) Immunoblot for snapin, SNAP-25, collectrin, EPAC2, VAMP2, and actin in 1: control mouse brain, 2: H4IIE (rat hepatoma) cells, 3: 3T3-L1 cells, 4: INS1 832/13 (rat insulinoma) cells, 5: MIN6 (mouse insulinoma) cells, 6: mouse islet protein extracts. Insulinoma cells and mouse islets express the proteins examined. Hepatoma cells express EPAC2 only, 3T3-L1 cells do not express the examined vesicle associated proteins. B) Immunohistochemical staining of mouse pancreas sections. Co-immunostaining with insulin (green) and with non-specific antibody (top) or snapin-specific antibody (bottom) (red). Nuclear counterstain with DAPI (blue). Separate pseudocolored images are shown with digitally merged image on bottom right panel, respectively. Snapin immunoreactivity co-localizes with insulin immunoreactivity in pancreatic islets. C) Co-immunoprecipitation for snapin serine phosphorylation in mouse islets treated with E4 without or with PKA specific inhibition with myr-PKI. E4 stimulates snapin phosphorylation, which is inhibited by adding myr-PKI. Immunoblot for 10% of protein input at bottom. D) Co-immunoprecipitation for snapin serine phosphorylation in human islets islets treated with E4 (10 nM) without or with PKA specific inhibition with myr-PKI. E4 stimulates snapin phosphorylation, which is inhibited by adding myr-PKI. Immunoblot for 10% of protein input at bottom. E) Snapin serine phosphorylation is increased in islets of Δ-prkar1a mice. Co-immunoprecipitation for snapin serine phosphorylation in wt-prkar1a and Δ-prkar1a islets.
Figure 5. PKA mediated phosphorylation of snapin maps to serine 50. Snapin S50 phosphorylation increases interaction with secretory vesicle-associated proteins SNAP-25, EPAC2 and collectrin. Snapin interaction with VAMP2 is glucose dependent. E4 stimulated SNAP-25 interaction with EPAC2 is not PKA mediated. Overexpression in islets of snapin S50D potentiates GSIS
A) Transiently transfected INS1 832/13 cells expressing C-terminal FLAG tagged WT, S50A or S50D snapin were treated with PBS (vehicle) E4 (10 nM), myr-PKI (10 nM), treated E4+myr-PKI, or transfected with Pkac followed by IP for FLAG or SNAP-25 and IB for phosphoserine or interacting proteins. Snapin serine phosphorylation occurs in WT snapin and not in S50A and S50D mutants by E4 and Pkac action. E4 effect is inhibited by myr-PKI. Snapin interaction with SNAP-25, collectrin or EPAC2 occurs only with phosphorylated WT snapin (by E4 or Pkac) or with snapin S50D as does SNAP-25 interaction with collectrin. SNAP-25 interaction with EPAC2 occurs with E4 in a PKA-independent manner and not inhibited by myr-PKI. B) Transiently transfected INS1 832/13 cells expressing C-terminal FLAG tagged WT, S50A or S50D snapin cultured in low (3 mM) or high (10 mM) glucose and co-IP/IB as in A. Snapin S50D mutant binds SNAP-25, collectrin and EPAC2 in both low and high glucose. Snapin interaction with VAMP2 occurs at elevated glucose levels only. SNAP-25- VAMP2 interaction occurs only with snapin S50D and at elevated glucose levels. C) Isolated C57Bl/6 mouse islets cultured in low (3 mM) or high (10 mM) glucose and treated with PBS, E4 (10 nM), myr-PKI (10 nM) and E4+myr-PKI followed by co-IP/IB as in A. Snapin serine phosphorylation is stimulated by E4 in a PKA dependent manner and increases snapin interaction with SNAP-25, collectrin and EPAC2 independently of glucose levels. Snapin-VAMP2 interaction is stimulated by E4 in PKA dependent manner only with high glucose. E4 stimulates SNAP25-EPAC2 interaction in a glucose- and PKA-independent manner. SNAP-25-VAMP2 interaction is stimulated by E4 in a glucose and PKA-dependent manner. PKA activity is verified by phosphorylation of CREB at serine 133 (p-CREB). D) Control and Δ-prkar1a islets cultured in low (3 mM) or high glucose (10 mM). IP for snapin or SNAP-25 and IB for phosphoserine and interacting proteins. Snapin phosphorylation and snapin interaction with SNAP-25, collectrin and EPAC2 are increased in Δ-prkar1a islets independently of glucose levels. Snapin-VAMP2 and SNAP-25-VAMP2 interactions are stimulated by high glucose only. E) Perifusion studies of C57Bl/6 mouse islets in low (3 mM) followed by high (10 mM) glucose concentrations. Islets were treated with either PBS (inverted triangle), E4 (10 nM) (upright triangle) during perifusion, or had been transduced with control (circle) or snapin S50D (square) expressing adenovirus. Table below curve summarizes area under the curves for first and second phase insulin secretion (*P<0.05 vs vehicle).
Figure 6. Snapin knockdown in C57Bl/6 mouse islets diminishes insulin secretion. Isolated mouse islets were transduced with lentivirus expressing shRNA with non-specific and snapin specific sequences
Left: Representative immunoblot of islet extracts shows 80% reduction of snapin protein 48 hours after viral transduction, SNAP25, collectrin, EPAC2 and VAMP2 remain unchanged. Right: Densitometric analysis of immunoblots from 3 different studies. B. Insulin secretion in static culture conditions from isolated islets with knocked-down snapin. In islets with reduced snapin, insulin secretion is diminished both at low (3 mM) and high (10 mM) glucose levels. Additional E4 administration does not significantly stimulate insulin secretion at low (3 mM) or high (10 mM) glucose conditions.
Figure 7. Impaired GSIS in DIO diabetic mouse islets is corrected with snapin S50D
A) Glucose and insulin levels of 12 week old C57Bl/6 littermate mice on either normal diet and after 6 weeks of high fat diet. Mice on high fat diet are hyperglycemic and have relative insulin deficiency. B) Immunoblot of islet proteins from C57Bl/6 mice on normal chow and high fat diet. C) Mapping of snapin serine-O-GlcNAcylation. DIO diabetic islets were transduced with adenovirus expressing C-terminal FLAG-tagged wild-type, S50A and S50D snapin isoforms. Immunoprecipitation with FLAG antibody followed by immunoblot was performed. Representative immunoblot is shown. Bar graph indicates densitometric analysis of 3 separate studies. D) Time course of snapin phosphorylation after E4 treatment of cultured islets of diet induced diabetic mice. E) Perifusion studies of DIO mouse islets in low (3 mM) followed by high (10 mM) glucose concentrations. Islets were treated with either PBS (inverted triangle), E4 (10 nM) (upright triangle) during perifusion, or had been transduced with control (circle) or snapin S50D (square) expressing adenovirus. Table below curve summarizes area under the curves for first and second phase insulin secretion. (*p<0.05 vs vehicle).
Similar articles
- Prkar1a in the regulation of insulin secretion.
Hussain MA, Stratakis C, Kirschner L. Hussain MA, et al. Horm Metab Res. 2012 Sep;44(10):759-65. doi: 10.1055/s-0032-1321866. Epub 2012 Sep 5. Horm Metab Res. 2012. PMID: 22951902 Free PMC article. Review. - SAD-A potentiates glucose-stimulated insulin secretion as a mediator of glucagon-like peptide 1 response in pancreatic β cells.
Nie J, Lilley BN, Pan YA, Faruque O, Liu X, Zhang W, Sanes JR, Han X, Shi Y. Nie J, et al. Mol Cell Biol. 2013 Jul;33(13):2527-34. doi: 10.1128/MCB.00285-13. Epub 2013 Apr 29. Mol Cell Biol. 2013. PMID: 23629625 Free PMC article. - SAD-A Promotes Glucose-Stimulated Insulin Secretion Through Phosphorylation and Inhibition of GDIα in Male Islet β Cells.
Nie J, Sun C, Chang Z, Musi N, Shi Y. Nie J, et al. Endocrinology. 2018 Aug 1;159(8):3036-3047. doi: 10.1210/en.2017-03243. Endocrinology. 2018. PMID: 29873699 Free PMC article. - Glucotoxicity inhibits cAMP-protein kinase A-potentiated glucose-stimulated insulin secretion in pancreatic β-cells.
Kong X, Yan D, Wu X, Guan Y, Ma X. Kong X, et al. J Diabetes. 2015 May;7(3):378-85. doi: 10.1111/1753-0407.12185. Epub 2014 Sep 6. J Diabetes. 2015. PMID: 24981285 - Molecular physiology of glucagon-like peptide-1 insulin secretagogue action in pancreatic β cells.
Leech CA, Dzhura I, Chepurny OG, Kang G, Schwede F, Genieser HG, Holz GG. Leech CA, et al. Prog Biophys Mol Biol. 2011 Nov;107(2):236-47. doi: 10.1016/j.pbiomolbio.2011.07.005. Epub 2011 Jul 19. Prog Biophys Mol Biol. 2011. PMID: 21782840 Free PMC article. Review.
Cited by
- Pancreatic β-cell response to increased metabolic demand and to pharmacologic secretagogues requires EPAC2A.
Song WJ, Mondal P, Li Y, Lee SE, Hussain MA. Song WJ, et al. Diabetes. 2013 Aug;62(8):2796-807. doi: 10.2337/db12-1394. Epub 2013 Apr 11. Diabetes. 2013. PMID: 23578994 Free PMC article. - Glucagon regulates hepatic kisspeptin to impair insulin secretion.
Song WJ, Mondal P, Wolfe A, Alonso LC, Stamateris R, Ong BW, Lim OC, Yang KS, Radovick S, Novaira HJ, Farber EA, Farber CR, Turner SD, Hussain MA. Song WJ, et al. Cell Metab. 2014 Apr 1;19(4):667-81. doi: 10.1016/j.cmet.2014.03.005. Cell Metab. 2014. PMID: 24703698 Free PMC article. - cAMP-independent effects of GLP-1 on β cells.
Kolic J, MacDonald PE. Kolic J, et al. J Clin Invest. 2015 Dec;125(12):4327-30. doi: 10.1172/JCI85004. Epub 2015 Nov 16. J Clin Invest. 2015. PMID: 26571393 Free PMC article. - Molecular mechanisms underlying physiological and receptor pleiotropic effects mediated by GLP-1R activation.
Pabreja K, Mohd MA, Koole C, Wootten D, Furness SG. Pabreja K, et al. Br J Pharmacol. 2014 Mar;171(5):1114-28. doi: 10.1111/bph.12313. Br J Pharmacol. 2014. PMID: 23889512 Free PMC article. Review. - Improved glucose tolerance with DPPIV inhibition requires β-cell SENP1 amplification of glucose-stimulated insulin secretion.
Ferdaoussi M, Smith N, Lin H, Bautista A, Spigelman AF, Lyon J, Dai X, Manning Fox JE, MacDonald PE. Ferdaoussi M, et al. Physiol Rep. 2020 Apr;8(8):e14420. doi: 10.14814/phy2.14420. Physiol Rep. 2020. PMID: 32339440 Free PMC article.
References
- Akimoto Y, Hart GW, Wells L, Vosseller K, Yamamoto K, Munetomo E, Ohara-Imaizumi M, Nishiwaki C, Nagamatsu S, Hirano H, et al. Elevation of the post-translational modification of proteins by O-linked N-acetylglucosamine leads to deterioration of the glucose-stimulated insulin secretion in the pancreas of diabetic Goto-Kakizaki rats. Glycobiology. 2007;17:127–140. - PubMed
- Boikos SA, Stratakis CA. Carney complex: pathology and molecular genetics. Neuroendocrinology. 2006;83:189–199. - PubMed
- Chepurny OG, Kelley GG, Dzhura I, Leech CA, Roe MW, Dzhura E, Li X, Schwede F, Genieser HG, Holz GG. PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM in human islets of Langerhans. Am J Physiol Endocrinol Metab. 2010;298:E622–633. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01 CA112268/CA/NCI NIH HHS/United States
- R01 DK081472-01A1/DK/NIDDK NIH HHS/United States
- P60 DK079637-05/DK/NIDDK NIH HHS/United States
- R01 DK081472-03/DK/NIDDK NIH HHS/United States
- P60 DK079637/DK/NIDDK NIH HHS/United States
- R01 DK081472-02/DK/NIDDK NIH HHS/United States
- R01 CA112268-05/CA/NCI NIH HHS/United States
- P30 DK079637/DK/NIDDK NIH HHS/United States
- RC4 DK090816/DK/NIDDK NIH HHS/United States
- R01 DK081472/DK/NIDDK NIH HHS/United States
- R24 DK084949/DK/NIDDK NIH HHS/United States
- R24 DK084949-01/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases