Phylogeographic origin of Helicobacter pylori is a determinant of gastric cancer risk - PubMed (original) (raw)
Multicenter Study
. 2011 Sep;60(9):1189-95.
doi: 10.1136/gut.2010.234468. Epub 2011 Feb 25.
M Blanca Piazuelo, Carrie L Shaffer, Barbara G Schneider, Mohammad Asim, Rupesh Chaturvedi, Luis E Bravo, Liviu A Sicinschi, Alberto G Delgado, Robertino M Mera, Dawn A Israel, Judith Romero-Gallo, Richard M Peek Jr, Timothy L Cover, Pelayo Correa, Keith T Wilson
Affiliations
- PMID: 21357593
- PMCID: PMC3133872
- DOI: 10.1136/gut.2010.234468
Multicenter Study
Phylogeographic origin of Helicobacter pylori is a determinant of gastric cancer risk
Thibaut de Sablet et al. Gut. 2011 Sep.
Abstract
Background and aims: Helicobacter pylori colonises the stomach in half of all humans, and is the principal cause of gastric cancer, the second leading cause of cancer death worldwide. While gastric cancer rates correlate with H pylori prevalence in some areas, there are regions where infection is nearly universal, but rates of gastric cancer are low. In the case of Colombia, there is a 25-fold increase in gastric cancer rate in the Andean mountain (high risk) region compared to the coastal (low risk) region, despite similarly high (∼90%) prevalence of H pylori in the two locations. Our aim was to investigate the ancestral origin of H pylori strains isolated from subjects in these high- and low-risk regions and to determine whether this is a predictive determinant of precancerous lesions.
Methods: Multi-locus sequence typing was used to investigate phylogeographic origins of infecting H pylori strains isolated from subjects in the Pacific coast and Andes Mountains in the state of Nariño, Colombia. We analysed 64 subjects infected with cagA+ vacA s1m1 strains. Gastric biopsy slides from each individual were scored for histological lesions and evaluated for DNA damage by immunohistochemistry.
Results: We show that strains from the high-risk region were all of European phylogeographic origin, whereas those from the low risk region were of either European (34%) or African origin (66%). European strain origin was strongly predictive of increased premalignant histological lesions and epithelial DNA damage, even in the low-risk region; African strain origin was associated with reduced severity of these parameters.
Conclusion: The phylogeographic origin of H pylori strains provides an explanation for geographic differences in cancer risk deriving from this infection.
Figures
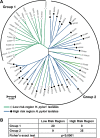
Figure 1
MLST analysis of 64 _cagA_+ vacA s1m1 Helicobacter pylori strains from Colombia. (A) Neighbor-joining tree; branches are drawn to scale to represent evolutionary distance. ◯ with green lines, isolates from LR region (low risk of gastric cancer); ● with blue lines, isolates from HR region (high risk of gastric cancer). The brackets denote the classification of the strains into two groups. (B) Distribution of strains into phylogenetic groups defined in (A), according to risk region of origin.
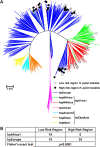
Figure 2
MLST analysis of 64 _cagA_+ vacA s1m1 Colombian isolates from the HR and LR regions, along with 380 reference strains that were previously classified into distinct ancestral haplogroups [34]. (A) Neighbor-joining tree built using bootstrapping with 10,000 trials. Branches are drawn to scale to represent evolutionary distance. ◯, isolates from LR region; ● isolates from HR region. The colours of the branches represent the classification of strains into distinct ancestral haplogroups [34]. (B) Distribution of strains into phylogeographic groups defined in (A), according to risk region of origin.
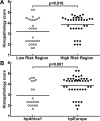
Figure 3
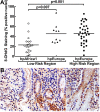
Histopathologic analysis of biopsies from subjects infected with Colombian H. pylori strains. Histologic scoring on a 1 to 6 scale (defined in Methods; each symbol represents the score for an individual subject. (A) Histopathology scores by risk region of origin. (B) Scores grouped by phylogenetic category of the strains. Horizontal bars represent the mean; p values were obtained by a linear multivariate model analysis.
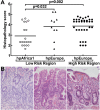
Figure 4
Histopathologic analysis of biopsies from subjects infected with Colombian H. pylori strains. Histologic scoring on a 1 to 6 scale (defined in Methods; each symbol represents the score for an individual subject. (A) Scores grouped according to phylogeny within a given risk region of origin. Horizontal bars represent the mean; pairwise comparisons were made using Dunnett's test with Sidak's adjustment. (B) Representative photomicrographs of hematoxylin and eosin staining of gastric tissue as follows: (left panel) LR subject infected with hpAfrica1 strain, showing non-atrophic gastritis; (center panel) LR subject infected with hpEurope strain, demonstrating intestinal metaplasia with changes indefinite for dysplasia; and (right panel) HR subject infected with hpEurope strain with intestinal metaplasia. Magnification in B was 200×.
Figure 5
Detection of oxidative DNA damage in gastric biopsies by quantification of 8-OHdG-positive epithelial cell nuclei, expressed as % positively-stained nuclei. (A) 8-OHdG levels, by risk region of origin. (B) Cases grouped according to strain phylogeny. Horizontal bars represent the mean; statistics were performed as in figure 3.
Figure 6
Detection of oxidative DNA damage in gastric biopsies by quantification of 8-OHdG-positive epithelial cell nuclei, expressed as % positively-stained nuclei. (A) Cases grouped according to strain phylogeny within a given risk region of origin. Horizontal bars represent the mean; statistics were performed as in figure 4. (B) Representative photomicrographs of 8-OHdG staining on gastric tissues as follows: (left panel) LR subject infected with hpAfrica1 strain; (center panel) LR subject infected with hpEurope strain; and (right panel) HR subject infected with hpEurope strain. Nuclei showing DNA damage are stained brown. Magnification in B was 400×.
Comment in
- Virulence factors or ancestral origin of Helicobacter pylori: which is a better predictor of gastric cancer risk?
Shiota S, Matsunari O, Watada M, Yamaoka Y. Shiota S, et al. Gut. 2012 Mar;61(3):469-70. doi: 10.1136/gutjnl-2011-300317. Epub 2011 May 24. Gut. 2012. PMID: 21610271 Free PMC article. No abstract available.
Similar articles
- Analysis of cagA in Helicobacter pylori strains from Colombian populations with contrasting gastric cancer risk reveals a biomarker for disease severity.
Loh JT, Shaffer CL, Piazuelo MB, Bravo LE, McClain MS, Correa P, Cover TL. Loh JT, et al. Cancer Epidemiol Biomarkers Prev. 2011 Oct;20(10):2237-49. doi: 10.1158/1055-9965.EPI-11-0548. Epub 2011 Aug 22. Cancer Epidemiol Biomarkers Prev. 2011. PMID: 21859954 Free PMC article. - Genetic polymorphisms in the cag pathogenicity island of Helicobacter pylori and risk of stomach cancer and high-grade premalignant gastric lesions.
Canzian F, Rizzato C, Obazee O, Stein A, Flores-Luna L, Camorlinga-Ponce M, Mendez-Tenorio A, Vivas J, Trujillo E, Jang H, Chen W, Kasamatsu E, Bravo MM, Torres J, Muñoz N, Kato I. Canzian F, et al. Int J Cancer. 2020 Nov 1;147(9):2437-2445. doi: 10.1002/ijc.33032. Epub 2020 May 14. Int J Cancer. 2020. PMID: 32363734 - Virulence of infecting Helicobacter pylori strains and intensity of mononuclear cell infiltration are associated with levels of DNA hypermethylation in gastric mucosae.
Schneider BG, Piazuelo MB, Sicinschi LA, Mera R, Peng DF, Roa JC, Romero-Gallo J, Delgado AG, de Sablet T, Bravo LE, Wilson KT, El-Rifai W, Peek RM Jr, Correa P. Schneider BG, et al. Epigenetics. 2013 Nov;8(11):1153-61. doi: 10.4161/epi.26072. Epub 2013 Aug 22. Epigenetics. 2013. PMID: 24128875 Free PMC article. - Helicobacter pylori-induced inflammation and epigenetic changes during gastric carcinogenesis.
Valenzuela MA, Canales J, Corvalán AH, Quest AF. Valenzuela MA, et al. World J Gastroenterol. 2015 Dec 7;21(45):12742-56. doi: 10.3748/wjg.v21.i45.12742. World J Gastroenterol. 2015. PMID: 26668499 Free PMC article. Review. - Mitochondrial DNA alterations in the progression of gastric carcinomas: unexplored issues and future research needs.
Rigoli L, Caruso RA. Rigoli L, et al. World J Gastroenterol. 2014 Nov 21;20(43):16159-66. doi: 10.3748/wjg.v20.i43.16159. World J Gastroenterol. 2014. PMID: 25473169 Free PMC article. Review.
Cited by
- Gastric cancer incidence and mortality is associated with altitude in the mountainous regions of Pacific Latin America.
Torres J, Correa P, Ferreccio C, Hernandez-Suarez G, Herrero R, Cavazza-Porro M, Dominguez R, Morgan D. Torres J, et al. Cancer Causes Control. 2013 Feb;24(2):249-56. doi: 10.1007/s10552-012-0114-8. Epub 2012 Dec 7. Cancer Causes Control. 2013. PMID: 23224271 Free PMC article. - Helicobacter pylori Antimicrobial Resistance and Gene Variants in High- and Low-Gastric-Cancer-Risk Populations.
Mannion A, Dzink-Fox J, Shen Z, Piazuelo MB, Wilson KT, Correa P, Peek RM Jr, Camargo MC, Fox JG. Mannion A, et al. J Clin Microbiol. 2021 Apr 20;59(5):e03203-20. doi: 10.1128/JCM.03203-20. Print 2021 Apr 20. J Clin Microbiol. 2021. PMID: 33692136 Free PMC article. - Aged Garlic and Cancer: A Systematic Review.
Miraghajani M, Rafie N, Hajianfar H, Larijani B, Azadbakht L. Miraghajani M, et al. Int J Prev Med. 2018 Sep 17;9:84. doi: 10.4103/ijpvm.IJPVM_437_17. eCollection 2018. Int J Prev Med. 2018. PMID: 30487964 Free PMC article. Review. - Study of Helicobacter pylori Isolated from a High-Gastric-Cancer-Risk Population: Unveiling the Comprehensive Analysis of Virulence-Associated Genes including Secretion Systems, and Genome-Wide Association Study.
Saruuljavkhlan B, Alfaray RI, Oyuntsetseg K, Gantuya B, Khangai A, Renchinsengee N, Matsumoto T, Akada J, Azzaya D, Davaadorj D, Yamaoka Y. Saruuljavkhlan B, et al. Cancers (Basel). 2023 Sep 12;15(18):4528. doi: 10.3390/cancers15184528. Cancers (Basel). 2023. PMID: 37760497 Free PMC article. - Phylogenomics of Colombian Helicobacter pylori isolates.
Gutiérrez-Escobar AJ, Trujillo E, Acevedo O, Bravo MM. Gutiérrez-Escobar AJ, et al. Gut Pathog. 2017 Sep 11;9:52. doi: 10.1186/s13099-017-0201-1. eCollection 2017. Gut Pathog. 2017. PMID: 28912838 Free PMC article.
References
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–5. - PubMed
- Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9. - PubMed
- Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–60. - PubMed
- Correa P, Haenszel W, Cuello C, et al. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res. 1990;50:4737–40. - PubMed
Publication types
MeSH terms
Grants and funding
- P30 DK058404-10/DK/NIDDK NIH HHS/United States
- R01 CA077955/CA/NCI NIH HHS/United States
- P01 CA116087-03/CA/NCI NIH HHS/United States
- R01CA77955/CA/NCI NIH HHS/United States
- KL2 RR024977/RR/NCRR NIH HHS/United States
- R01 DK053620-10/DK/NIDDK NIH HHS/United States
- R01 DK058587-09/DK/NIDDK NIH HHS/United States
- P01 CA116087/CA/NCI NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- R01 DK058587/DK/NIDDK NIH HHS/United States
- R01 CA077955-13/CA/NCI NIH HHS/United States
- P01CA116087/CA/NCI NIH HHS/United States
- UL1 RR024975-04/RR/NCRR NIH HHS/United States
- R01 DK053620/DK/NIDDK NIH HHS/United States
- P01 CA028842/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R01 AT004821-03/AT/NCCIH NIH HHS/United States
- R01AI068009/AI/NIAID NIH HHS/United States
- R01 AT004821/AT/NCCIH NIH HHS/United States
- TL1 RR024978/RR/NCRR NIH HHS/United States
- UL1RR024975/RR/NCRR NIH HHS/United States
- R01AI039657/AI/NIAID NIH HHS/United States
- P30DK058404/DK/NIDDK NIH HHS/United States
- R01 AI039657/AI/NIAID NIH HHS/United States
- R01 AI039657-14/AI/NIAID NIH HHS/United States
- R01 AI068009/AI/NIAID NIH HHS/United States
- R01DK58587/DK/NIDDK NIH HHS/United States
- R01DK053620/DK/NIDDK NIH HHS/United States
- R01 AI068009-03/AI/NIAID NIH HHS/United States
- P01 CA028842-24/CA/NCI NIH HHS/United States
- P01CA028842/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical