Grand rounds at the National Institutes of Health: HDAC inhibitors as radiation modifiers, from bench to clinic - PubMed (original) (raw)
Review
Grand rounds at the National Institutes of Health: HDAC inhibitors as radiation modifiers, from bench to clinic
Jacob E Shabason et al. J Cell Mol Med. 2011 Dec.
Abstract
Glioblastoma multiforme (GBM) is the most common and aggressive malignant brain tumour. Patients afflicted with this disease unfortunately have a very poor prognosis, and fewer than 5% of patients survive for 5 years from the time of diagnosis. Therefore, improved therapies to treat this disease are sorely needed. One such class of drugs that have generated great enthusiasm for the treatment of numerous malignancies, including GBM, is histone deacetylase (HDAC) inhibitors. Pre-clinical data have demonstrated the efficacy of various HDAC inhibitors as anticancer agents, with the greatest effects shown when HDAC inhibitors are used in combination with other therapies. As a result of encouraging pre-clinical data, numerous HDAC inhibitors are under investigation in clinical trials, either as monotherapies or in conjunction with other treatments such as chemotherapy, biologic therapy or radiation therapy. In fact, two actively studied HDAC inhibitors, vorinostat and depsipeptide, were recently approved for the treatment of refractory cutaneous T cell lymphoma. In this review, we first present a patient with GBM, and then discuss the pathogenesis, epidemiology and current treatment options of GBM. Finally, we examine the translation of pre-clinical studies that have demonstrated HDAC inhibitors as potent radiosensitizers in in vitro and in vivo models, to a phase II clinical trial combining the HDAC inhibitor, valproic acid, along with temozolomide and radiation therapy for the treatment of GBM.
Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd No claim to US government works.
Figures
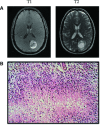
Fig 1
(A) T1 and T2 representative MRI images of the patient at diagnosis. These images display a ring-enhancing lesion in the left parietal occipital lobe, characteristic of GBM. (B) Representative pathological image of GBM displaying pseudopalisading formation of malignant cells surrounding areas of necrosis (Frontalcortex.com).
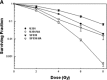
Fig 2
Excerpt graphs from Camphausen et al. showing in vitro and in vivo radiosensitization of glioma cells by valproic acid treatment (28). (A) U251 and SF539 glioma cells were treated with valproic acid both before and following radiation. Clonogenic survival curves reveal an increase in radiosensitivity in valproic acid treated cells. (B) The effects of valproic acid and radiation on tumour growth delay. U251 glioma cells were implanted into the hind leg of mice and divided into four treatment groups: (1) control, (2) 4 Gy radiation, (3) valproic acid, (4) valproic acid and 4 Gy radiation. The combination treatment regimen showed the most significant tumour growth delay.
Fig 2
Excerpt graphs from Camphausen et al. showing in vitro and in vivo radiosensitization of glioma cells by valproic acid treatment (28). (A) U251 and SF539 glioma cells were treated with valproic acid both before and following radiation. Clonogenic survival curves reveal an increase in radiosensitivity in valproic acid treated cells. (B) The effects of valproic acid and radiation on tumour growth delay. U251 glioma cells were implanted into the hind leg of mice and divided into four treatment groups: (1) control, (2) 4 Gy radiation, (3) valproic acid, (4) valproic acid and 4 Gy radiation. The combination treatment regimen showed the most significant tumour growth delay.
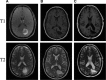
Fig 3
A series of T1 and T2 weighted Brain MRIs from the presented patient with GBM. (A) Represents images from the initial diagnosis. (B) Represents images shortly after gross surgical resection, showing typical post-surgical changes. (C) Represents the most recent MRI images with no evidence of disease recurrence 3 years after the initial diagnosis of GBM.
Similar articles
- HDAC inhibitors in cancer care.
Tofilon PJ, Camphausen K. Tofilon PJ, et al. Oncology (Williston Park). 2010 Feb;24(2):180-5. Oncology (Williston Park). 2010. PMID: 20361469 Free PMC article. - Histone deacetylase inhibitors in glioblastoma: pre-clinical and clinical experience.
Bezecny P. Bezecny P. Med Oncol. 2014 Jun;31(6):985. doi: 10.1007/s12032-014-0985-5. Epub 2014 May 18. Med Oncol. 2014. PMID: 24838514 Review. - Enhanced efficacy of histone deacetylase inhibitor combined with bromodomain inhibitor in glioblastoma.
Meng W, Wang B, Mao W, Wang J, Zhao Y, Li Q, Zhang C, Tang Y, Ma J. Meng W, et al. J Exp Clin Cancer Res. 2018 Oct 1;37(1):241. doi: 10.1186/s13046-018-0916-y. J Exp Clin Cancer Res. 2018. PMID: 30285808 Free PMC article. - Inhibition of LSD1 sensitizes glioblastoma cells to histone deacetylase inhibitors.
Singh MM, Manton CA, Bhat KP, Tsai WW, Aldape K, Barton MC, Chandra J. Singh MM, et al. Neuro Oncol. 2011 Aug;13(8):894-903. doi: 10.1093/neuonc/nor049. Epub 2011 Jun 8. Neuro Oncol. 2011. PMID: 21653597 Free PMC article. - Advances in histone deacetylase inhibitors in targeting glioblastoma stem cells.
Reddy RG, Bhat UA, Chakravarty S, Kumar A. Reddy RG, et al. Cancer Chemother Pharmacol. 2020 Aug;86(2):165-179. doi: 10.1007/s00280-020-04109-w. Epub 2020 Jul 7. Cancer Chemother Pharmacol. 2020. PMID: 32638092 Review.
Cited by
- Histone deacetylase inhibitor, valproic acid, radiosensitizes the C6 glioma cell line in vitro.
Zhou Y, Xu Y, Wang H, Niu J, Hou H, Jiang Y. Zhou Y, et al. Oncol Lett. 2014 Jan;7(1):203-208. doi: 10.3892/ol.2013.1666. Epub 2013 Nov 7. Oncol Lett. 2014. PMID: 24348849 Free PMC article. - The challenges and the promise of molecular targeted therapy in malignant gliomas.
Wang H, Xu T, Jiang Y, Xu H, Yan Y, Fu D, Chen J. Wang H, et al. Neoplasia. 2015 Mar;17(3):239-55. doi: 10.1016/j.neo.2015.02.002. Neoplasia. 2015. PMID: 25810009 Free PMC article. Review. - NAMPT as a Dedifferentiation-Inducer Gene: NAD+ as Core Axis for Glioma Cancer Stem-Like Cells Maintenance.
Lucena-Cacace A, Umeda M, Navas LE, Carnero A. Lucena-Cacace A, et al. Front Oncol. 2019 May 2;9:292. doi: 10.3389/fonc.2019.00292. eCollection 2019. Front Oncol. 2019. PMID: 31119097 Free PMC article. Review. - Glioblastoma: Current Status, Emerging Targets, and Recent Advances.
Thakur A, Faujdar C, Sharma R, Sharma S, Malik B, Nepali K, Liou JP. Thakur A, et al. J Med Chem. 2022 Jul 14;65(13):8596-8685. doi: 10.1021/acs.jmedchem.1c01946. Epub 2022 Jul 5. J Med Chem. 2022. PMID: 35786935 Free PMC article. Review. - Early phase clinical studies of AR-42, a histone deacetylase inhibitor, for neurofibromatosis type 2-associated vestibular schwannomas and meningiomas.
Welling DB, Collier KA, Burns SS, Oblinger JL, Shu E, Miles-Markley BA, Hofmeister CC, Makary MS, Slone HW, Blakeley JO, Mansouri SA, Neff BA, Jackler RK, Mortazavi A, Chang LS. Welling DB, et al. Laryngoscope Investig Otolaryngol. 2021 Aug 20;6(5):1008-1019. doi: 10.1002/lio2.643. eCollection 2021 Oct. Laryngoscope Investig Otolaryngol. 2021. PMID: 34667843 Free PMC article.
References
- Central Brain Tumor Registry of the United States. 2010. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2006. Source: Central Brain Tumor Registry of the United States, Hinsdale, IL. Website: http://www.cbtrus.org/2010-NPCR-SEER/CBTRUS-WEBREPORT-Final-3-2-10.pdf.
- Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528–37. - PubMed
- Ron E, Modan B, Boice JD, Jr, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319:1033–9. - PubMed
- Ironside JW, Weller RO, Moss TH. Astrocytic tumours. In: Ironside JW, Moss TH, Louis DN, Lowe JS, Weller RO, et al., editors. Diagnostic pathology of nervous system tumours. London: Churchill Livingstone; 2002. pp. 88–96.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials