Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress - PubMed (original) (raw)
Review
Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress
Ira Tabas et al. Nat Cell Biol. 2011 Mar.
Abstract
The ability to respond to perturbations in endoplasmic reticulum (ER) function is a fundamentally important property of all cells, but ER stress can also lead to apoptosis. In settings of chronic ER stress, the associated apoptosis may contribute to pathophysiological processes involved in a number of prevalent diseases, including neurodegenerative diseases, diabetes, atherosclerosis and renal disease. The molecular mechanisms linking ER stress to apoptosis are the topic of this review, with emphases on relevance to pathophysiology and integration and complementation among the various apoptotic pathways induced by ER stress.
© 2011 Macmillan Publishers Limited. All rights reserved
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures
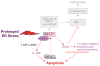
Figure 1
Prolonged activation of IRE1 may promote apoptosis. Studies with cultured cells have identified a pro-apoptotic IRE1–TRAF2–JNK pathway that can be activated by prolonged ER stress. Signal transduction between IRE1–TRAF2 and phosphorylation of JNK may be mediated in certain settings by the MAP kinase kinase kinase (MAPKKK) ASK1 and its activator, AIP1. JNK-induced apoptosis may involve the pro-apoptotic Bcl-2 family members, Bax and Bak, which in turn, can amplify the IRE1 signal. Prolonged IRE1-mediated activation of the RIDD pathway may promote apoptosis by degrading mRNAs encoding essential cell-survival proteins, including XPB1 itself.
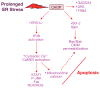
Figure 2
Pathways through which prolonged activation of CHOP may promote apoptosis. Studies with both cultured cells and gene-targeted mouse models have identified a number of possible pro-apoptotic actions of prolonged CHOP expression, as depicted here and explained in detail in the text. Note that two of the major cell death pathways — the ERO1α–IP3R–Ca2+–CaMKII pathway and the Bcl-2 family member pathway — may lead to a common pro-apoptotic endpoint of outer mitochondrial membrane (OMM) permeabilization (dotted arrow; see text for details).
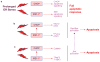
Figure 3
Examples of integration among the UPR apoptosis pathways. (a) In the simplest scenario, differential apoptotic signalling downstream of CHOP and IRE1 may lead to only partial cell death responses, and so the full apoptotic response may require activation of both pathways. (b) Both pathways can promote changes in Bcl-2 family protein expression or activity that favour cell death. (c) Both pathways can promote JNK activation, which when prolonged, can trigger cell death. Note that one of the mechanisms by which activated JNK leads to apoptosis is through Bax–Bak activation, possibly leading to further integration (purple dotted arrow). See text for details and for other possible areas of integration.
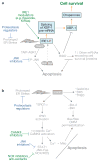
Figure 4
Examples of therapeutic strategies to prevent cell death in the setting of pathologic, prolonged ER stress. (a, b) Strategies directed towards IRE1- and CHOP-mediated apoptotic pathways, respectively. Note that several of the approaches are applicable to both (blue font). The common approaches include mitigation of ER stress itself by so-called proteostasis regulators and inhibition of JNK. See text for details.
Similar articles
- Glycogen synthase kinase-3 regulates endoplasmic reticulum (ER) stress-induced CHOP expression in neuronal cells.
Meares GP, Mines MA, Beurel E, Eom TY, Song L, Zmijewska AA, Jope RS. Meares GP, et al. Exp Cell Res. 2011 Jul 1;317(11):1621-8. doi: 10.1016/j.yexcr.2011.02.012. Epub 2011 Feb 26. Exp Cell Res. 2011. PMID: 21356208 Free PMC article. - Oxidative stress mediated Ca(2+) release manifests endoplasmic reticulum stress leading to unfolded protein response in UV-B irradiated human skin cells.
Farrukh MR, Nissar UA, Afnan Q, Rafiq RA, Sharma L, Amin S, Kaiser P, Sharma PR, Tasduq SA. Farrukh MR, et al. J Dermatol Sci. 2014 Jul;75(1):24-35. doi: 10.1016/j.jdermsci.2014.03.005. Epub 2014 Apr 13. J Dermatol Sci. 2014. PMID: 24794973 - The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection.
Hu H, Tian M, Ding C, Yu S. Hu H, et al. Front Immunol. 2019 Jan 4;9:3083. doi: 10.3389/fimmu.2018.03083. eCollection 2018. Front Immunol. 2019. PMID: 30662442 Free PMC article. Review. - Endoplasmic reticulum stress mediates withaferin A-induced apoptosis in human renal carcinoma cells.
Choi MJ, Park EJ, Min KJ, Park JW, Kwon TK. Choi MJ, et al. Toxicol In Vitro. 2011 Apr;25(3):692-8. doi: 10.1016/j.tiv.2011.01.010. Epub 2011 Jan 23. Toxicol In Vitro. 2011. PMID: 21266191 - The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress.
Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl JA, Majsterek I. Rozpedek W, et al. Curr Mol Med. 2016;16(6):533-44. doi: 10.2174/1566524016666160523143937. Curr Mol Med. 2016. PMID: 27211800 Free PMC article. Review.
Cited by
- Endoplasmic reticulum stress as a novel target to ameliorate epithelial-to-mesenchymal transition and apoptosis of human peritoneal mesothelial cells.
Shin HS, Ryu ES, Oh ES, Kang DH. Shin HS, et al. Lab Invest. 2015 Oct;95(10):1157-73. doi: 10.1038/labinvest.2015.91. Epub 2015 Jul 20. Lab Invest. 2015. PMID: 26192086 - Unfolded Protein Response in Leukemia: From Basic Understanding to Therapeutic Opportunities.
Khateb A, Ronai ZA. Khateb A, et al. Trends Cancer. 2020 Nov;6(11):960-973. doi: 10.1016/j.trecan.2020.05.012. Epub 2020 Jun 13. Trends Cancer. 2020. PMID: 32540455 Free PMC article. Review. - Molecular imaging of apoptosis: from micro to macro.
Zeng W, Wang X, Xu P, Liu G, Eden HS, Chen X. Zeng W, et al. Theranostics. 2015 Feb 20;5(6):559-82. doi: 10.7150/thno.11548. eCollection 2015. Theranostics. 2015. PMID: 25825597 Free PMC article. Review. - Emodin-Induced Oxidative Inhibition of Mitochondrial Function Assists BiP/IRE1_α_/CHOP Signaling-Mediated ER-Related Apoptosis.
Qiu LZ, Yue LX, Ni YH, Zhou W, Huang CS, Deng HF, Wang NN, Liu H, Liu X, Zhou YQ, Xiao CR, Wang YG, Gao Y. Qiu LZ, et al. Oxid Med Cell Longev. 2021 Apr 22;2021:8865813. doi: 10.1155/2021/8865813. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33968299 Free PMC article. - Irisin Ameliorate Acute Pancreatitis and Acinar Cell Viability through Modulation of the Unfolded Protein Response (UPR) and PPARγ-PGC1α-FNDC5 Pathways.
Horwitz A, Birk R. Horwitz A, et al. Biomolecules. 2024 May 30;14(6):643. doi: 10.3390/biom14060643. Biomolecules. 2024. PMID: 38927047 Free PMC article.
References
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
- Ron D, Hubbard SR. How IRE1 reacts to ER stress. Cell. 2008;132:24–26. - PubMed
- Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. - PubMed
- Ma Y, Hendershot LM. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem. 2003;278:34864–34873. - PubMed
- Seo HY, et al. Endoplasmic reticulum stress-induced activation of activating transcription factor 6 decreases cAMP-stimulated hepatic gluconeogenesis via inhibition of CREB. Endocrinology. 2010;151:561–568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL054591/HL/NHLBI NIH HHS/United States
- HL054591/HL/NHLBI NIH HHS/United States
- HL087123/HL/NHLBI NIH HHS/United States
- R01 HL075662/HL/NHLBI NIH HHS/United States
- HL075662/HL/NHLBI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- P01 HL087123/HL/NHLBI NIH HHS/United States
- R01 HL106019/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources