Differential regulation of dendritic and axonal development by the novel Krüppel-like factor Dar1 - PubMed (original) (raw)
Comparative Study
Differential regulation of dendritic and axonal development by the novel Krüppel-like factor Dar1
Bing Ye et al. J Neurosci. 2011.
Abstract
Dendrites and axons are two major neuronal compartments with differences that are critical for neuronal functions. To learn about the differential regulation of dendritic and axonal development, we conducted a genetic screen in Drosophila and isolated the dendritic arbor reduction 1 (dar1) mutants, which display defects in dendritic but not axonal growth. The dar1 gene encodes a novel transcription regulator in the Krüppel-like factor family. Neurons lacking dar1 function have severely reduced growth of microtubule- but not F-actin-based dendritic branches. In contrast, overexpression of Dar1 dramatically increased the growth of microtubule-based dendritic branches. Our results suggest that Dar1 promotes dendrite growth in part by suppressing the expression of the microtubule-severing protein Spastin. Our study thus uncovers a novel transcriptional program for microtubule regulation that preferentially controls dendrite growth.
Figures
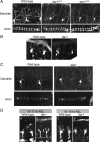
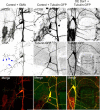
Figure 1.
dar1 specifically controls dendrite development. A, The class IV da neurons display reduced dendritic growth but normal axonal growth in both dar13010 and dar13232 mutant embryos. Dendrites and axons were live-imaged with the class IV da neuron-specific marker _ppk_-eGFP. The dendritic morphology of single class IV neurons is shown in the top panels, and the axonal terminals of these neurons, located in the ventral nerve cord, are show in the bottom panels. The ladder-like structures of the axons are formed by all class IV neurons (6 in each segment). B, The class I da neurons display reduced dendritic growth in dar1 mutant embryos. Dendrites were delineated with the marker GAL42-21, UAS-mCD8-GFP. Because of the lack of a specific marker for class I da neurons, axon morphology of these neurons cannot be assessed in embryos. C, Dar1 mutant embryos without any potential maternal and zygotic contributions of Dar1 display the same dendrite and axon phenotype as zygotic dar1 mutants. The class IV da neurons were visualized with the _ppk_-eGFP marker. D, The dendrite phenotype of dar1 mutants is a result of defective outgrowth. Dendrite defects were visible at 16–17 h AEL shortly after the initiation of dendrite growth, the earliest time point that can be imaged with the marker GAL4109-2-80, UAS-mCD8-GFP, and became more pronounced in 18–19 h AEL.
Figure 2.
dar1 functions cell-autonomously to specifically control dendrite development. A, MARCM clones of da neurons homozygous for dar1 mutation. a–d, Control neurons. e–h, dar1 mutant neurons. B, Axonal growth of dar1 mutant neurons is normal. The axons of the dar1 mutant neurons not only extended normally from the body wall to the ventral nerve cord, which is ∼1700 μm away (Ye et al., 2007), but also developed terminals that are indistinguishable from those of wild-type neurons. Shown in the figure are the axonal terminals of a wild-type and a dar1 mutant class IV neuron ddaC in abdominal segment 6.
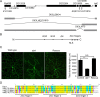
Figure 3.
dar1 encodes a novel Krüppel-like factor. A, Genome view of the dar1 locus. Both Df(3L)GN34 and Df(3L)ΔEY01819 failed to complement lethality and dendrite defects, whereas Df(3L)R75 complemented both lethality and dendrite defects. The distal breakpoint of Df(3L)GN34 lies in between CG14974 and CG12029, and the proximal breakpoint of Df(3L)ΔEY01819 is in encore. The distal breakpoint of Df(3L)R75 lies in between CG32264 and CG10855. These results suggest that dar1 mutations lie in between CG14974 and CG10855. Recombination mapping with P-element insertions suggested that Dar1 is 0.08 cM proximal to the P-element insertion of EY11305 (in Eip63E), 0.5 cM distal to KG07161 (in CG12006), and 0.6 cM distal to EY01819 (in encore). B, The domain scheme of the protein product of CG12029. There are three zinc fingers in the C-terminal domain preceded by a NLS. dar13232, dar13010, and dar1D6 each carries a unique nonsense mutation in CG12029, which leads to truncation of the coding sequence [marked by an asterisk (*)]. C, A genomic-cDNA transgene rescues the dendrite defects of dar1 mutants. Homozygous dar1 mutant larvae carrying the rescue transgene were imaged at early third-instar stage. The bar chart presents the quantification of dendrite length in early third-instar larvae. **p < 0.01; N.S., not significant (_p_ > 0.05, t test). Error bars indicate SEM. D, Amino acid sequence comparison of the zinc fingers of Dar1, mouse KLF5 (mKLF5), mouse KLF7 (mKLF7), and mouse (mKLF9). The numbers in the parentheses after the mouse KLFs indicate sequence similarity between each mouse KLF and Dar1. Residues identical in all four proteins are marked with yellow background. Those conserved in some of the four proteins are shown in light blue background. Residuals similar to the conserved ones are shown in pink background. Dar1 and mKLF5 also share a stretch of amino acids upstream of the zinc finger region, which is not shown here.
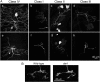
Figure 4.
Localization of Dar1 protein in wild-type and dar1 mutant embryos. Stage 13 embryos were stained with antibodies against Dar1 (green) and neuron-specific transcription factor Elav (magenta). A, Immunostaining of a wild-type embryo showing that Dar1 protein is present in the PNS but absent in the CNS. B, Immunostaining of the dorsal cluster of the PNS. Dar1 is present in the nuclei of neurons extending more than one dendrite (the da neurons, dbd neuron, and dmd1 neuron) but is absent in neurons having a single dendrite (es neurons). C, Cytoplasmic rather than nuclear Dar1 immunoreactivity in dar1D6 mutant embryos carrying a premature stop codon preceding the coding sequences for the NLS and zinc fingers. The immunosignal in each neuron occupies a larger area than that of the nuclear staining in wild-type animals.
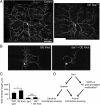
Figure 5.
dar1 is not required for forming F-actin-enriched dendritic filopodia. A, Representative images showing that dar1 mutations do not block filopodium formation induced by overexpressing Rac1. GAL42-21 was used to drive the expression of mCD8-GFP and Rac1 in class I da neurons. B, Quantification of terminal branch number per 100 μm of major dendrites. Note that class I neurons in dar1 mutant embryos display increased number of terminal branches that are transient as they disappear in larval stages and are not visible in MARCM clones. *p < 0.05, t test. Error bars indicate SEM. C, Overexpression of Dar1 results in overgrowth of dendrites. Representative images of control and Dar1-overexpressing (“OE Dar1”) class IV da neuron. GAL44-77 was used to express mCD8-GFP and Dar1 in class IV da neurons. Images were collected from early third-instar larvae. D, Sholl analysis of the dendritic morphology of control and Dar1-overexpressing class IV da neurons. Diamond, Control neurons; dot, Dar1-overexpressing neurons.
Figure 6.
Dar1 promotes microtubule-based dendritic growth. F-actin marker GMA (green channel in left column) and microtubule marker tubulin-GFP (green channel in middle and right columns) were expressed in the class III neuron ddaA together with the membrane marker mCD8-mRFP (red channel in all three columns) for dendritic morphology by the driver GAL419-12. The blue arrowheads point to several examples of the dendritic spikes, which are enriched with GMA (F-actin) but lack tubulin-GFP. Overexpression of dar1 leads to elongated branches that contain tubulin-GFP.
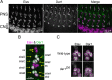
Figure 7.
Upregulation of the microtubule-severing protein Spastin in dar1 mutant neurons may be responsible for the dendrite defects. A, Representative images showing that upregulation of Spastin by overexpressing SpaT32 reduced both total dendritic length and branch numbers in class IV da neurons. B, Dar1 is essential for the transcription factor Knot, which promotes Spastin expression, to promote dendritic growth in class I da neurons. The MARCM technique was used to overexpress Knot in single class I da neurons. Representative images are shown. Overexpressing Knot causes dendritic overgrowth in wild-type class I da neurons (left), and dar1 mutations abolish the effects of Knot overexpression (right). C, Quantification of the total dendritic length in class I neurons overexpressing Knot, those defective of dar1, and those overexpressing Knot in dar1 mutant background. Error bars indicate SEM. *p < 0.01, t test. D, A model that explains how the Dar1–Spastin pathway differentiates dendrite and axon growth. Dar1 suppresses Spastin expression, either directly or indirectly, in addition to positively control the expression of an inhibitory mechanism of microtubule severing (e.g., MAPs or microtubule posttranslational modification) specifically in the axon. Spastin is capable of severing microtubules in both dendrites and axons. Whether the microtubule severing by Spastin promotes or inhibits dendrite/axon growth depends on the expression levels of Spastin.
Similar articles
- The Krüppel-Like Factor Dar1 Determines Multipolar Neuron Morphology.
Wang X, Zhang MW, Kim JH, Macara AM, Sterne G, Yang T, Ye B. Wang X, et al. J Neurosci. 2015 Oct 21;35(42):14251-9. doi: 10.1523/JNEUROSCI.1610-15.2015. J Neurosci. 2015. PMID: 26490864 Free PMC article. - Katanin p60-like1 promotes microtubule growth and terminal dendrite stability in the larval class IV sensory neurons of Drosophila.
Stewart A, Tsubouchi A, Rolls MM, Tracey WD, Sherwood NT. Stewart A, et al. J Neurosci. 2012 Aug 22;32(34):11631-42. doi: 10.1523/JNEUROSCI.0729-12.2012. J Neurosci. 2012. PMID: 22915107 Free PMC article. - The krüppel-like factor Dar1 restricts the proliferation of Drosophila intestinal stem cells.
Wu X, Chen Z, Gao Y, Wang L, Sun X, Jin Y, Liu W. Wu X, et al. FEBS J. 2018 Nov;285(21):3945-3958. doi: 10.1111/febs.14652. Epub 2018 Sep 25. FEBS J. 2018. PMID: 30188612 - Nmnat exerts neuroprotective effects in dendrites and axons.
Wen Y, Parrish JZ, He R, Zhai RG, Kim MD. Wen Y, et al. Mol Cell Neurosci. 2011 Sep;48(1):1-8. doi: 10.1016/j.mcn.2011.05.002. Epub 2011 May 9. Mol Cell Neurosci. 2011. PMID: 21596138 Free PMC article. - Knot/Collier and cut control different aspects of dendrite cytoskeleton and synergize to define final arbor shape.
Jinushi-Nakao S, Arvind R, Amikura R, Kinameri E, Liu AW, Moore AW. Jinushi-Nakao S, et al. Neuron. 2007 Dec 20;56(6):963-78. doi: 10.1016/j.neuron.2007.10.031. Neuron. 2007. PMID: 18093520
Cited by
- The Drosophila homolog of APP promotes Dscam expression to drive axon terminal growth, revealing interaction between Down syndrome genes.
Pizzano S, Sterne GR, Veling MW, Xu LA, Hergenreder T, Ye B. Pizzano S, et al. Dis Model Mech. 2023 Sep 1;16(9):dmm049725. doi: 10.1242/dmm.049725. Epub 2023 Sep 15. Dis Model Mech. 2023. PMID: 37712356 Free PMC article. - The Krüppel-Like Factor Dar1 Determines Multipolar Neuron Morphology.
Wang X, Zhang MW, Kim JH, Macara AM, Sterne G, Yang T, Ye B. Wang X, et al. J Neurosci. 2015 Oct 21;35(42):14251-9. doi: 10.1523/JNEUROSCI.1610-15.2015. J Neurosci. 2015. PMID: 26490864 Free PMC article. - isoTarget: A Genetic Method for Analyzing the Functional Diversity of Splicing Isoforms In Vivo.
Liu H, Pizzano S, Li R, Zhao W, Veling MW, Hu Y, Yang L, Ye B. Liu H, et al. Cell Rep. 2020 Nov 10;33(6):108361. doi: 10.1016/j.celrep.2020.108361. Cell Rep. 2020. PMID: 33176150 Free PMC article. - Neuronal polarity: demarcation, growth and commitment.
Cáceres A, Ye B, Dotti CG. Cáceres A, et al. Curr Opin Cell Biol. 2012 Aug;24(4):547-53. doi: 10.1016/j.ceb.2012.05.011. Epub 2012 Jun 20. Curr Opin Cell Biol. 2012. PMID: 22726583 Free PMC article. Review. - Trim9 regulates activity-dependent fine-scale topography in Drosophila.
Yang L, Li R, Kaneko T, Takle K, Morikawa RK, Essex L, Wang X, Zhou J, Emoto K, Xiang Y, Ye B. Yang L, et al. Curr Biol. 2014 May 5;24(9):1024-30. doi: 10.1016/j.cub.2014.03.041. Epub 2014 Apr 17. Curr Biol. 2014. PMID: 24746793 Free PMC article.
References
- Baas PW. Microtubules and neuronal polarity: lessons from mitosis. Neuron. 1999;22:23–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 MH080599-03/MH/NIMH NIH HHS/United States
- K99 MH080599/MH/NIMH NIH HHS/United States
- R37 NS040929/NS/NINDS NIH HHS/United States
- R00 MH080599/MH/NIMH NIH HHS/United States
- R01 MH091186-01/MH/NIMH NIH HHS/United States
- R01 NS047200-01/NS/NINDS NIH HHS/United States
- K99 MH080599-02/MH/NIMH NIH HHS/United States
- R01NS47200/NS/NINDS NIH HHS/United States
- R00 MH080599-04/MH/NIMH NIH HHS/United States
- R01MH091186/MH/NIMH NIH HHS/United States
- HHMI_/Howard Hughes Medical Institute/United States
- K99 MH080599-01/MH/NIMH NIH HHS/United States
- R00MH080599/MH/NIMH NIH HHS/United States
- R01 MH084234/MH/NIMH NIH HHS/United States
- R01 MH091186/MH/NIMH NIH HHS/United States
- R00 MH080599-05/MH/NIMH NIH HHS/United States
- R37NS40929/NS/NINDS NIH HHS/United States
- R37 NS040929-10/NS/NINDS NIH HHS/United States
- R01 NS047200/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous