Eradication of solid tumors using histone deacetylase inhibitors combined with immune-stimulating antibodies - PubMed (original) (raw)
Eradication of solid tumors using histone deacetylase inhibitors combined with immune-stimulating antibodies
Ailsa J Christiansen et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Histone deacetylase inhibitors (HDACi) have been successfully used as monotherapies for the treatment of hematological malignancies; however, the single agent effects of HDACi against solid tumors are less robust. Using preclinical models of lymphoma, we have recently demonstrated that HDACi induce tumor cell-specific apoptosis and that this is essential for the therapeutic effects of these agents. Herein, we demonstrate that HDACi can be combined with immune-activating antibodies designed to promote the function of antigen-presenting cells (APCs) and enhance proliferation and survival of cytotoxic T cells (CTL) to stimulate a host antitumor immune response resulting in eradication of established solid tumors. This unique combination therapy was dependent on tumor cell apoptosis mediated by HDACi that stimulated the uptake of dead tumor cells by APCs. Tumor eradication was mediated by CD8(+) CTL that used perforin as the key immune effector molecule. This combination therapy was well tolerated and induced long-term immunological antitumor memory capable of mediating spontaneous tumor eradication upon rechallenge. These studies indicate that the ability of HDACi to mediate subtherapeutic levels of tumor cell apoptosis can be exploited by combining with antibodies that augment host antitumor immune responses to mediate robust and prolonged eradication of solid tumors.
Conflict of interest statement
Conflict of interest statement: The R.W.J. laboratory has collaborative research grants from Merck & Co and Novartis for studies involving vorinostat and panobinostat, respectively.
Figures
Fig. 1.
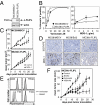
V/bimAb therapy induces the regression of established s.c. carcinoma of diverse tissue origins. (A) Cohorts of mice with established (>9 mm2) s.c. carcinoma (4T1.2, MC38, or Renca) were treated i.p. with 150 mg/kg vorinostat daily for 7 d. The dose was then decreased to 100 mg/kg for the following 8 d. BimAb (100 μg anti-CD137, 25 μg anti-CD40) was administered every 4 d for 4 doses. Mice were treated with either vorinostat and bimAb (V/bimAb, n = 8), bimAb or vorinostat alone (n = 6), or vehicle with isotype mAb (control, n = 6). Tumor growth was assessed every 2–3 d; mean tumor size ± SEM are shown. (B) Therapeutic efficacy of V/bimAb was also assessed in Rag2−/−c-γ-chain−/− and Rag1/J mice with established MC38 carcinomas. (C) Mice that achieved complete tumor regressions (MC38, from A) when treated with V/bimAb were rechallenged on the opposite hind flank with 1 × 106 MC38 and tumor growth assessed compared with naïve C57BL/6 mice. Tumors spontaneously regressed in 5/9 rechallenge mice, demonstrating V/bimAb therapy facilitates the generation of long-term immunological memory. Data shown is representative of at least two independent experiments. Enhancement of therapeutic efficacy comparing bimAb alone and in combination with vorinostat and single agent activity compared with control was assessed using the Mann-Whitney test; *P < 0.05.
Fig. 2.
Vorinostat-induced apoptosis is required for therapeutic efficacy of V/bimAb. (A) MC38 carcinomas retrovirally transduced to overexpress murine Bcl-2 (MC38/Bcl-2) were assessed for overexpression of exogenous protein by Western blotting. (B) Empty vector control cells (MC38/MSCV) and MC38/Bcl-2 cells were assessed for in vitro sensitivity to vorinostat following culture for 48 h. Data shown are the mean ± SEM of three independent experiments. (C) Tumor cell apoptosis following treatment of MC38/MSCV and MC38/Bcl-2 cells with 5 μM and 25 μM vorinostat for 24 h was assessed using annexinV/propidium iodide staining and flow cytometry. Representative flow cytometry profiles are shown. (D) Engulfment of CFSE-labeled vorinostat-treated (24 h) MC38/MSCV and MC38/Bcl-2 cells by bone marrow-derived CD11c+ APCs was assessed by flow cytometry. Bcl-2 overexpression blocked uptake of MC38 carcinomas following treatment with vorinostat. (E and F) To confirm apoptosis correlated with engulfment by APCs, a time course was performed. Annexin V-positive staining of vorinostat-treated MC38/MSCV and MC38/Bcl-2 cells are shown in E, with the corresponding median fluorescence intensity in FL-1 (i.e., CFSE) following co-culture of tumor cells with APCs, gated on CD11c positive cells shown in F. Data shown are the mean ± SEM of three independent experiments. (G) Efficacy of V/bimAb therapy was then assessed in mice bearing established MC38/Bcl-2 carcinomas. Cohorts of mice with established (>9 mm2) s.c. MC38/Bcl-2 carcinoma were treated with vehicle (n = 6), vorinostat (n = 6), bimAb (n = 6), or V/bimAb (n = 8) using the therapeutic schedule described in Fig. 1. Tumor growth was assessed every 2–3 d; mean tumor size ± SEM are shown. No complete tumor regressions were observed in mice in any of the treatment groups. Data shown are representative of two independent experiments.
Fig. 3.
V/bimAb therapy is efficacious against established TRAIL-resistant carcinomas. (A) MC38 carcinomas retrovirally transduced to overexpress murine c-FLIPL were assessed for overexpression of exogenous protein by Western blotting. (B) MC38/MSCV and MC38/c-FLIPL cells were treated with increasing concentrations of vorinostat for 48 h or plate-bound MD5-1 for 24 h and apoptosis was assessed using annexin V staining and flow cytometry. Data shown are the mean ± SEM of three independent experiments; statistical significance was assessed using the Student's t test. *P < 0.05. (_C_) Mice with established MC38/MSCV or MC38/c-FLIPL carcinomas were treated with isotype control or MD5-1 mAb (4 doses, every 4 d, 50 μg per dose, _n_ = 6 per treatment group). Tumor growth was assessed every 2–3 d; mean tumor size ± SEM are shown. (_D_) TRAIL pathway sensitivity was also assessed in situ via TUNEL staining of established tumors taken from MD5-1 and control-treated mice (tissue harvested 8 h post-mAb (50 μg) treatment). (_E_) The expression of cell surface DR5, TRAIL, Fas, and FasL was assessed on untreated and vorinostat-treated MC38 cells by flow cytometry. (_F_) Efficacy of V/bimAb therapy was assessed in mice bearing established MC38/c-FLIPL carcinomas. Cohorts of mice with established (>9 mm2) s.c. MC38/c-FLIPL carcinoma were treated with vehicle (n = 6), vorinostat (n = 6), bimAb (n = 6), or V/bimAb (n = 8) using the therapeutic schedule described in Fig. 1. Tumor growth was assessed every 2–3 d; mean tumor size ± SEM are shown and are representative of two independent experiments. Efficacy of MD5-1, V/bimAb, and the single agent activity compared with control treatment was assessed using the Mann-Whitney test. *P < 0.05.
Fig. 4.
Role of immune-specific effector cells and regulatory proteins in mediating V/bimAb therapy. (A) Cohorts of mice with established (>9 mm2) s.c. MC38 tumors were treated with anti-CD4 (GK1.5), anti-CD8 (53-6.7), or anti-asialo GM1 to deplete and/or inactivate CD4+, CD8+, and NK cells, respectively. Cell lineage-deficient tumor-bearing mice were then treated with either vehicle (n = 6) or V/bimAb (n = 8) using the therapeutic schedule described in Fig. 1. Tumor growth was assessed every 2–3 d and tumor size in individual mice (mm2) is shown. Data shown are representative of two independent experiments. (B) Cohorts of knockout mice deficient in IFNγ (IFN-γ−/−), TRAIL (TRAIL−/−), or perforin (pfp−/−) bearing established MC38 carcinomas (>9 mm2) were treated with either vehicle (n = 6) or V/bimAb (n = 8) using the therapeutic schedule described in Fig. 1. Tumor growth was assessed every 2–3 d and tumor size in individual mice (mm2) is shown. Data shown are representative of two independent experiments.
Similar articles
- An intact immune system is required for the anticancer activities of histone deacetylase inhibitors.
West AC, Mattarollo SR, Shortt J, Cluse LA, Christiansen AJ, Smyth MJ, Johnstone RW. West AC, et al. Cancer Res. 2013 Dec 15;73(24):7265-76. doi: 10.1158/0008-5472.CAN-13-0890. Epub 2013 Oct 24. Cancer Res. 2013. PMID: 24158093 - Antitumor activities and on-target toxicities mediated by a TRAIL receptor agonist following cotreatment with panobinostat.
Martin BP, Frew AJ, Bots M, Fox S, Long F, Takeda K, Yagita H, Atadja P, Smyth MJ, Johnstone RW. Martin BP, et al. Int J Cancer. 2011 Jun 1;128(11):2735-47. doi: 10.1002/ijc.25594. Epub 2010 Oct 13. Int J Cancer. 2011. PMID: 20715169 - Combination therapy of established cancer using a histone deacetylase inhibitor and a TRAIL receptor agonist.
Frew AJ, Lindemann RK, Martin BP, Clarke CJ, Sharkey J, Anthony DA, Banks KM, Haynes NM, Gangatirkar P, Stanley K, Bolden JE, Takeda K, Yagita H, Secrist JP, Smyth MJ, Johnstone RW. Frew AJ, et al. Proc Natl Acad Sci U S A. 2008 Aug 12;105(32):11317-22. doi: 10.1073/pnas.0801868105. Epub 2008 Aug 6. Proc Natl Acad Sci U S A. 2008. PMID: 18685088 Free PMC article. - Histone deacetylase inhibitors: an overview of the clinical studies in solid tumors.
Slingerland M, Guchelaar HJ, Gelderblom H. Slingerland M, et al. Anticancer Drugs. 2014 Feb;25(2):140-9. doi: 10.1097/CAD.0000000000000040. Anticancer Drugs. 2014. PMID: 24185382 Review. - Histone deacetylase inhibitors in the treatment of hematological malignancies.
Petrella A, Fontanella B, Carratù A, Bizzarro V, Rodriquez M, Parente L. Petrella A, et al. Mini Rev Med Chem. 2011 Jun;11(6):519-27. doi: 10.2174/138955711795843347. Mini Rev Med Chem. 2011. PMID: 21561404 Review.
Cited by
- In vivo treatment with epigenetic modulating agents induces transcriptional alterations associated with prognosis and immunomodulation in multiple myeloma.
Maes K, De Smedt E, Kassambara A, Hose D, Seckinger A, Van Valckenborgh E, Menu E, Klein B, Vanderkerken K, Moreaux J, De Bruyne E. Maes K, et al. Oncotarget. 2015 Feb 20;6(5):3319-34. doi: 10.18632/oncotarget.3207. Oncotarget. 2015. PMID: 25669970 Free PMC article. - HDACs and HDAC Inhibitors in Cancer Development and Therapy.
Li Y, Seto E. Li Y, et al. Cold Spring Harb Perspect Med. 2016 Oct 3;6(10):a026831. doi: 10.1101/cshperspect.a026831. Cold Spring Harb Perspect Med. 2016. PMID: 27599530 Free PMC article. Review. - Phase I/Ib Study of Pembrolizumab Plus Vorinostat in Advanced/Metastatic Non-Small Cell Lung Cancer.
Gray JE, Saltos A, Tanvetyanon T, Haura EB, Creelan B, Antonia SJ, Shafique M, Zheng H, Dai W, Saller JJ, Chen Z, Tchekmedyian N, Goas K, Thapa R, Boyle TA, Chen DT, Beg AA. Gray JE, et al. Clin Cancer Res. 2019 Nov 15;25(22):6623-6632. doi: 10.1158/1078-0432.CCR-19-1305. Epub 2019 Aug 13. Clin Cancer Res. 2019. PMID: 31409616 Free PMC article. Clinical Trial. - Epigenetics in Cancer: A Hematological Perspective.
Stahl M, Kohrman N, Gore SD, Kim TK, Zeidan AM, Prebet T. Stahl M, et al. PLoS Genet. 2016 Oct 10;12(10):e1006193. doi: 10.1371/journal.pgen.1006193. eCollection 2016 Oct. PLoS Genet. 2016. PMID: 27723796 Free PMC article. Review. - Immune effectors required for the therapeutic activity of vorinostat.
Kepp O, Galluzzi L, Kroemer G. Kepp O, et al. Oncoimmunology. 2013 Nov 1;2(11):e27157. doi: 10.4161/onci.27157. Epub 2013 Nov 13. Oncoimmunology. 2013. PMID: 24475375 Free PMC article. No abstract available.
References
- Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. - PubMed
- Ghiringhelli F, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. - PubMed
- Aymeric L, et al. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010;70:855–858. - PubMed
- Ma Y, et al. Chemotherapy and radiotherapy: Cryptic anticancer vaccines. Semin Immunol. 2010;22:113–124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous