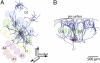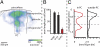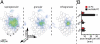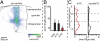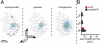Three-dimensional axon morphologies of individual layer 5 neurons indicate cell type-specific intracortical pathways for whisker motion and touch - PubMed (original) (raw)
Three-dimensional axon morphologies of individual layer 5 neurons indicate cell type-specific intracortical pathways for whisker motion and touch
Marcel Oberlaender et al. Proc Natl Acad Sci U S A. 2011.
Abstract
The cortical output layer 5 contains two excitatory cell types, slender- and thick-tufted neurons. In rat vibrissal cortex, slender-tufted neurons carry motion and phase information during active whisking, but remain inactive after passive whisker touch. In contrast, thick-tufted neurons reliably increase spiking preferably after passive touch. By reconstructing the 3D patterns of intracortical axon projections from individual slender- and thick-tufted neurons, filled in vivo with biocytin, we were able to identify cell type-specific intracortical circuits that may encode whisker motion and touch. Individual slender-tufted neurons showed elaborate and dense innervation of supragranular layers of large portions of the vibrissal area (total length, 86.8 ± 5.5 mm). During active whisking, these long-range projections may modulate and phase-lock the membrane potential of dendrites in layers 2 and 3 to the whisking cycle. Thick-tufted neurons with soma locations intermingling with those of slender-tufted ones display less dense intracortical axon projections (total length, 31.6 ± 14.3 mm) that are primarily confined to infragranular layers. Based on anatomical reconstructions and previous measurements of spiking, we put forward the hypothesis that thick-tufted neurons in rat vibrissal cortex receive input of whisker motion from slender-tufted neurons onto their apical tuft dendrites and input of whisker touch from thalamic neurons onto their basal dendrites. During tactile-driven behavior, such as object location, near-coincident input from these two pathways may result in increased spiking activity of thick-tufted neurons and thus enhanced signaling to their subcortical targets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Three-dimensional reconstructions of in vivo filled slender-tufted pyramidal neuron in L5 of rat vibrissal cortex. (A) View on cortical surface (dorsal axis points out of the paper plane). Neuronal processes (axon, blue; basal dendrites, red; apical dendrites, orange) are shown with reference to the barrel field in L4 (letters A–E refer to whisker rows, numbers refer to whiskers within the same row). Note the wide lateral spread and dense innervation of slender-tufted axon into multiple barrel columns surrounding the principal column, which contains the cell's soma. In addition, this neuron displays long-range projections outside the vibrissal area into surrounding higher-order dysgranular cortices (DZ). (B) Semicoronal view along whisker row D in A. Note that the wide-ranging lateral projections are primarily confined to supragranular layers. Projections in dysgranular cortex originate from a single branch and end in clusters of columnar dimensions.
Fig. 2.
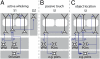
Quantification of 3D intracortical axon innervation of slender-tufted neurons (I). (A) Semicoronal view of 3D axon density of the five neurons shown in
Fig. S1
. The dashed box renders the approximate dimensions of the respective principal column of each neuron tracing. Note that innervation is not restricted to the principal column (PC), particularly in supragranular layers. (B) Most axon is found outside the principal column, or even extends into dysgranular cortices outside the vibrissal area, which cannot be recovered in in vitro preparations. (C) Axon length profiles along the vertical column axis. Within the principal column, L5 slender-tufted neurons display two innervation zones: one in supragranular L2/L3 and one in infragranular L5; outside the principal column innervation is largely restricted to supragranular layers, indicating that L5 slender-tufted–L5 projections remain local (i.e., intracolumnar), whereas L5 slender-tufted–L2/L3 projections represent primarily long-range projections (i.e., leaving the principal column).
Fig. 3.
Quantification of 3D intracortical axon innervation of slender-tufted neurons (II). (A) Tangential view of Fig. 2_A_ in supragranular, granular, and infragranular layers. Projections from L5 slender-tufted neurons remain largely confined to the principal column in granular and infragranular layers, but innervate almost the entire vibrissal area in supragranular layers. (B) Quantification of axon length within and outside the principal column reveals that most axon is found in supragranular layers outside the principal column.
Fig. 4.
Three-dimensional reconstructions of in vivo filled thick-tufted pyramidal neuron in L5 of rat vibrissal cortex. (A) Tangential view and notation as in Fig. 1_A_. Note that the intracortical axon innervation by thick-tufted cells is far less elaborate compared with slender-tufted neurons. Further, innervation remains largely confined to the principal column and a limited number of surrounding columns in its immediate vicinity. (B) Semicoronal view as in Fig. 1_A_. Please note that the horizontal projections to surrounding columns remain primarily confined to infragranular layers, whereas projections to granular and supragranular layers remain within the lateral boundaries of the principal column.
Fig. 5.
Quantification of 3D intracortical axon innervation of thick-tufted neurons (I). (A) Semicoronal view of 3D axon density of the five neurons shown in
Fig. S2
. Please note that innervation in granular and supragranular layers respects the lateral borders of the principal column, whereas innervation in infragranular layers extends to surrounding columns. (B) Thick-tufted neurons display much less axon compared with slender-tufted neurons (Fig. 2_B_). Whereas the amount of axon is similar for slender-tufted and thick-tufted neurons within the principal column (16.4 ± 6.7 vs. 10.1 ± 6.4; P = 0.17), long-range projections are significantly less elaborate for thick-tufted neurons (59.6 ± 9.7 vs. 18.6 ± 7.5; P < 0.0001). (C) The axon length profile of thick-tufted neurons within the principal column reveals that intracolumnar innervation is almost homogeneous throughout all layers, reaching a peak in infragranular L5 approximately 1,250 μm below the pia surface. The profile outside the principal column shows that long-range projections are confined to infragranular layers.
Fig. 6.
Quantification of 3D intracortical axon innervation of thick-tufted neurons (II). (A) Tangential view of Fig. 5_A_ in supragranular, granular, and infragranular layers. Projections of thick-tufted neurons remain almost completely confined to the lateral column boundaries in granular and supragranular layers, but spread to adjacent columns in infragranular layers. (B) Quantification of axon length within and outside the principal column reveals that most axon is found in infragranular layers outside the principal column.
Fig. 7.
Cell type-specific microcircuits in L5 are involved during different behavioral states. (A) During active whisking, slender-tufted neurons display spiking activity that carries phase information (p). Because of their intracortical axon projection pattern, slender-tufted neurons from a single column convey this information (i) via local circuits to L5 neurons within the principal column (not shown), yielding whisker-specific output of slender-tufted neurons to the striatum; and (ii) via long-range circuits to supragranular layers of the entire vibrissal area and adjacent dysgranular zones, diminishing whisker specificity in supragranular layers. (B) During passive whisker touch, thick-tufted neurons display the highest increase in spiking activity in vibrissal cortex, primarily caused by thalamocortical input from VPM (t). Because of their 3D intracortical axon pattern, thick-tufted neurons from a single cortical column convey information of whisker touch (i) via local circuits to all layers within the principal column (not shown), (ii) via long-range circuits to L5 of surrounding columns, and (iii) to subcortical targets (e.g., POm and pons). (C) Object location during active whisker motion may be encoded by simultaneous input from slender-tufted neurons to apical tufts and from VPM to basal dendrites of L5 thick-tufted neurons, causing increased spiking activity (bold arrow) and enhanced output to subcortical targets (e.g., corticothalamic feedback to POm).
Similar articles
- High-frequency burst spiking in layer 5 thick-tufted pyramids of rat primary somatosensory cortex encodes exploratory touch.
de Kock CPJ, Pie J, Pieneman AW, Mease RA, Bast A, Guest JM, Oberlaender M, Mansvelder HD, Sakmann B. de Kock CPJ, et al. Commun Biol. 2021 Jun 10;4(1):709. doi: 10.1038/s42003-021-02241-8. Commun Biol. 2021. PMID: 34112934 Free PMC article. - Pyramidal neurons in layer 5 of the rat visual cortex. I. Correlation among cell morphology, intrinsic electrophysiological properties, and axon targets.
Kasper EM, Larkman AU, Lübke J, Blakemore C. Kasper EM, et al. J Comp Neurol. 1994 Jan 22;339(4):459-74. doi: 10.1002/cne.903390402. J Comp Neurol. 1994. PMID: 8144741 - Spiking in primary somatosensory cortex during natural whisking in awake head-restrained rats is cell-type specific.
de Kock CP, Sakmann B. de Kock CP, et al. Proc Natl Acad Sci U S A. 2009 Sep 22;106(38):16446-50. doi: 10.1073/pnas.0904143106. Epub 2009 Sep 4. Proc Natl Acad Sci U S A. 2009. PMID: 19805318 Free PMC article. - The vibrissal system as a model of thalamic operations.
Deschênes M, Timofeeva E, Lavallée P, Dufresne C. Deschênes M, et al. Prog Brain Res. 2005;149:31-40. doi: 10.1016/S0079-6123(05)49003-2. Prog Brain Res. 2005. PMID: 16226574 Review. - A night in the life of a rat: vibrissal mechanics and tactile exploration.
Hartmann MJ. Hartmann MJ. Ann N Y Acad Sci. 2011 Apr;1225:110-8. doi: 10.1111/j.1749-6632.2011.06007.x. Ann N Y Acad Sci. 2011. PMID: 21534998 Review.
Cited by
- Layer 1 of somatosensory cortex: an important site for input to a tiny cortical compartment.
Ledderose JMT, Zolnik TA, Toumazou M, Trimbuch T, Rosenmund C, Eickholt BJ, Jaeger D, Larkum ME, Sachdev RNS. Ledderose JMT, et al. Cereb Cortex. 2023 Nov 27;33(23):11354-11372. doi: 10.1093/cercor/bhad371. Cereb Cortex. 2023. PMID: 37851709 Free PMC article. - Shared and divergent principles of synaptic transmission between cortical excitatory neurons in rodent and human brain.
de Kock CPJ, Feldmeyer D. de Kock CPJ, et al. Front Synaptic Neurosci. 2023 Sep 5;15:1274383. doi: 10.3389/fnsyn.2023.1274383. eCollection 2023. Front Synaptic Neurosci. 2023. PMID: 37731775 Free PMC article. Review. - Spatiotemporal spike-centered averaging reveals symmetry of temporal and spatial components of the spike-LFP relationship during human focal seizures.
Lee S, Deshpande SS, Merricks EM, Schlafly E, Goodman R, McKhann GM, Eskandar EN, Madsen JR, Cash SS, van Putten MJAM, Schevon CA, van Drongelen W. Lee S, et al. Commun Biol. 2023 Mar 25;6(1):317. doi: 10.1038/s42003-023-04696-3. Commun Biol. 2023. PMID: 36966217 Free PMC article. - Different subtypes of motor cortex pyramidal tract neurons projects to red and pontine nuclei.
Lopez-Virgen V, Olivares-Moreno R, de Lafuente V, Concha L, Rojas-Piloni G. Lopez-Virgen V, et al. Front Cell Neurosci. 2022 Dec 20;16:1073731. doi: 10.3389/fncel.2022.1073731. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36605617 Free PMC article. - Distinct organization of two cortico-cortical feedback pathways.
Shen S, Jiang X, Scala F, Fu J, Fahey P, Kobak D, Tan Z, Zhou N, Reimer J, Sinz F, Tolias AS. Shen S, et al. Nat Commun. 2022 Oct 27;13(1):6389. doi: 10.1038/s41467-022-33883-9. Nat Commun. 2022. PMID: 36302912 Free PMC article.
References
- Hallman LE, Schofield BR, Lin CS. Dendritic morphology and axon collaterals of corticotectal, corticopontine, and callosal neurons in layer V of primary visual cortex of the hooded rat. J Comp Neurol. 1988;272:149–160. - PubMed
- Jones EG. Cerebral cortex. Laminar Distribution of Cortical Efferent Cells. In: Peters A, Jones EG, editors. New York: Plenum; 1984. pp. 521–553.
- Alloway KD. Information processing streams in rodent barrel cortex: the differential functions of barrel and septal circuits. Cereb Cortex. 2008;18:979–989. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources